Ghs Reference Materials Health Hazard Criteria
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Chemical Hygiene Plan Manual
CHEMICAL HYGIENE PLAN AND HAZARDOUS MATERIALS SAFETY MANUAL FOR LABORATORIES This is the Chemical Hygiene Plan specific to the following areas: Laboratory name or room number(s): ___________________________________ Building: __________________________________________________________ Supervisor: _______________________________________________________ Department: _______________________________________________________ Telephone numbers 911 for Emergency and urgent consultation 48221 Police business line 46919 Fire Dept business line 46371 Radiological and Environmental Management Revisied on: Enter a revision date here. All laboratory chemical use areas must maintain a work-area specific Chemical Hygiene Plan which conforms to the requirements of the OSHA Laboraotry Standard 29 CFR 19190.1450. Purdue University laboratories may use this document as a starting point for creating their work area specific CHP. Minimally this cover page is to be edited for work area specificity (non-West Lafayette laboratories are to place their own emergency, fire, and police telephone numbers in the space above) AND appendix K must be completed. This instruction and information box should remain. This model CHP is version 2010A; updates are to be found at www.purdue.edu/rem This page intentionally blank. PURDUE CHEMICAL HYGIENE PLAN AWARENESS CERTIFICATION For CHP of: ______________________________ Professor, building, rooms The Occupational Safety and Health Administration (OSHA) requires that laboratory employees be made aware of the Chemical Hygiene Plan at their place of employment (29 CFR 1910.1450). The Purdue University Chemical Hygiene Plan and Hazardous Materials Safety Manual serves as the written Chemical Hygiene Plan (CHP) for laboratories using chemicals at Purdue University. The CHP is a regular, continuing effort, not a standby or short term activity. Departments, divisions, sections, or other work units engaged in laboratory work whose hazards are not sufficiently covered in this written manual must customize it by adding their own sections as appropriate (e.g. -

Argonne Report.Pdf
CONTENTS NOTATION ........................................................................................................................... xi ABSTRACT ........................................................................................................................... 1 1 INTRODUCTION ........................................................................................................... 5 1.1 Overview of the Emergency Response Guidebook ................................................ 5 1.2 Organization of this Report ..................................................................................... 7 2 GENERAL METHODOLOGY ....................................................................................... 9 2.1 TIH List ................................................................................................................... 10 2.1.1 Background ................................................................................................. 10 2.1.2 Changes in the TIH List for the ERG2012 ................................................. 11 2.2 Shipment and Release Scenarios ............................................................................ 11 2.2.1 Shipment Profiles ........................................................................................ 12 2.2.2 Treatment of Chemical Agents ................................................................... 14 2.3 Generics, Mixtures, and Solutions .......................................................................... 17 2.4 Analysis of Water-Reactive -

Particularly Hazardous Substances
Particularly Hazardous Substances In its Laboratory Standard, OSHA requires the establishment of additional protections for persons working with "Particularly Hazardous Substances" (PHS). OSHA defines these materials as "select" carcinogens, reproductive toxins and acutely toxic materials. Should you wish to add: explosive, violently reactive, pyrophoric and water-reactve materials to this category, the information is included. Carbon nanotubes have also been added due to their suspected carcinogenic properties. This table is designed to assist the laboratory in the identification of PHS, although it is not definitively conclusive or entirely comprehensive. *Notes on the proper use of this table appear on page 12. 1 6 5 2 3 4 Substance CAS National Toxicity National Program Carcinogen Toxin Acute Regulated OSHA Carcinogen Group IARC Carcinogen Toxin Reproductive Violently Reactive/ Explosive/Peroxide Forming/Pyrophoric A-a-C(2-Amino-9H-pyrido[2,3,b]indole) 2648-68-5 2B Acetal 105-57-7 yes Acetaldehyde 75-07-0 NTP AT 2B Acrolein (2-Propenal) 107-02-8 AT Acetamide 126850-14-4 2B 2-Acetylaminofluorene 53-96-3 NTP ORC Acrylamide 79-06-6 NTP 2B Acrylyl Chloride 814-68-6 AT Acrylonitrile 107-13-1 NTP ORC 2B Adriamycin 23214-92-8 NTP 2A Aflatoxins 1402-68-2 NTP 1 Allylamine 107-11-9 AT Alkylaluminums varies AT Allyl Chloride 107-05-1 AT ortho-Aminoazotoluene 97-56-3 NTP 2B para-aminoazobenzene 60-09-3 2B 4-Aminobiphenyl 92-67-1 NTP ORC 1 1-Amino-2-Methylanthraquinone 82-28-0 NTP (2-Amino-6-methyldipyrido[1,2-a:3’,2’-d]imidazole) 67730-11-4 2B -

United States Patent Office Patented Mar
3,171,249 United States Patent Office Patented Mar. 2, 1965 i 2 fuel rocket engine. The above and other objects of this 3,171,249 PROPELLANT AND Rick |PROPULSSON METH invention will become apparent from the discussion OXD EMPLOYANG EYERAZINE WITH AMSNO which follows. TETRAZOLES The objects of this invention are accomplished by the Ronald E. Be, Canoga Park, Calif., assigner to use of compounds having the general formula: North American Aviatiosa, Bac. No Drawing. Fied Nov. 29, 1961, Ser. No. 155,803 NH2. (R) 8 Clains. (C. 60-35.4) N This invention relates to a novel rocket propellant. YS More particularly, this invention relates to a novel in 10 N--H proved rocket propellant and a method of operating a wherein x varies from 0 to 1 and R is selected from the rocket engine. class consisting of HCl, H2O, HNO3, and HCIO, as addi The criterion by which rocket propellants are classi tives to a hydrazine-based rocket fuel in an amount suffi fied is specific impulse, Is, defined as thrust in pounds 5 cient to depress the freezing point at least 40° C. while divided by the total mass flow of fuel and oxidizer in retaining about the same density impulse and specific pounds per second. Specific impulse is thus given in impulse. Hence, an embodiment of this invention com units of "seconds.” Oxidizer-fuel propulsion system prises a method of operating a rocket engine comprising compositions with a relatively high specific impulse are ejecting from the reaction chamber of the engine a gaseous known in the art. -

How to Identify and Report Hazardous Substances on The
OREGON COMMUNITY RIGHT TO KNOW AND PROTECTION ACT How to Identify and Report Hazardous Substances on the Hazardous Substance Information Survey February 2012 For assistance call the Mailing Address: Hazardous Substance Office of State Fire Marshal Information Hotline Community Right to Know Unit 4760 Portland Rd NE (503) 378-6835 Salem, OR 97305-1760 Toll Free (800) 454-6125 TDD (503) 390-4661 Monday – Friday Website: 8AM – 12PM and 1PM – 5PM http://www.oregon.gov/OSP/SFM/CR2K_Home.shtml Visit our website for more information: http://www.oregon.gov/OSP/SFM/CR2K_Home.shtml. These documents are currently available from our website: Blank Section D Chemical Form Blank Section E Additional Storage Location Form Survey Request Form Gas Conversion Chart Survey Mailing Schedule TABLE OF CONTENTS Introduction…………………………………………………………….. 1 Quick Steps to Complete the Survey …………………………………. 1 Reporting Requirements ……………………………...................... 2 What is a Hazardous Substance? ……………………………………... 3 What is a Reportable Quantity? ……………………………………….3 Reporting Compressed Gases ……………………………………….. 4 Liquefied and Cryogenic Gases …………………………………........ 4 Reporting Lead Acid Batteries ……………………………………….. 5 Tables for Completing the Survey ……………………………………... 6 Instructions and Definitions ……………………………………………. 7 Reporting Storage Locations …………………………………………..11 Frequently Asked Questions ………………………………………….. 13 EHS, 112r, PSM Questions …………………………………………… 14 EHS List ..............................................................................15 112(r) List ............................................................................18 -

Detection of Cyanotoxins, Β-N-Methylamino-L-Alanine and Microcystins, from a Lake Surrounded by Cases of Amyotrophic Lateral Sclerosis
Toxins 2015, 7, 322-336; doi:10.3390/toxins7020322 OPEN ACCESS toxins ISSN 2072-6651 www.mdpi.com/journal/toxins Article Detection of Cyanotoxins, β-N-methylamino-L-alanine and Microcystins, from a Lake Surrounded by Cases of Amyotrophic Lateral Sclerosis Sandra Anne Banack 1, Tracie Caller 2,†, Patricia Henegan 3,†, James Haney 4,†, Amanda Murby 4, James S. Metcalf 1, James Powell 1, Paul Alan Cox 1 and Elijah Stommel 3,* 1 Institute for Ethnomedicine, PO Box 3464, Jackson, WY 83001, USA; E-Mails: [email protected] (S.A.B.); [email protected] (J.S.M.); [email protected] (J.P.); [email protected] (P.A.C.) 2 Cheyenne Regional Medical Group, Cheyenne, WY 82001, USA; E-Mail: [email protected] 3 Department of Neurology, Dartmouth-Hitchcock Medical Center, Lebanon, NH 03756, USA; E-Mail: [email protected] 4 Department of Biological Sciences, University of New Hampshire, Durham, NH 03824, USA; E-Mails: [email protected] (J.H.); [email protected] (A.M.) † These authors contributed equally to this work. * Author to whom correspondence should be addressed; E-Mail: [email protected]; Tel.: +1-603-650-8615; Fax: +1-603-650-6233. Academic Editor: Luis M. Botana Received: 19 November 2014 / Accepted: 21 January 2015 / Published: 29 January 2015 Abstract: A cluster of amyotrophic lateral sclerosis (ALS) has been previously described to border Lake Mascoma in Enfield, NH, with an incidence of ALS approximating 25 times expected. We hypothesize a possible association with cyanobacterial blooms that can produce β-N-methylamino-L-alanine (BMAA), a neurotoxic amino acid implicated as a possible cause of ALS/PDC in Guam. -

FIREDOC Vocabulary List, 3Rd Edition
FIREDOC Vocabulary List, 3rd Edition II^STT United states Department of Commerce XI I National Institute of Standards and Technology NATIONAL INSTITUTE OF STANDARDS & TECHNOLOGY Research Information Center Gaithersburg, MD 20899 DATE DUE Demco, inc. 38-293 NIST Special Publication 779 FIREDOC Vocabulary List, 3rd Edition Nora H. Jason Center for Fire Research National Engineering Laboratory National Institute of Standards and Technology Gaithersburg, MD 20899 (Supersedes NBSIR 85-3231 and NBSIR 87-3545) February 1990 U.S. Department of Commerce Robert A. Mosbacher, Secretary National Institute of Standards and Technology John W. Lyons, Director National Institute of Standards U.S. Government Printing Office For sale by the Superintendent and Technology Washington: 1990 of Documents Special Publication 779 U.S. Government Printing Office (Supersedes NBSIR 85-3231 Washington, DC 20402 and NBSIR 87-3545) Natl. Inst. Stand. Technol. Spec. Publ. 779 104 pages (Feb. 1990) CODEN: NSPUE2 Contents Acknowledgments v Introduction 1 Symbol Notation 1 Terms Beginning with A 3 Terms Beginning with B 11 Terms Beginning with C 15 Terms Beginning with D 23 Terms Beginning with E 29 Terms Beginning with F 33 Terms Beginning with G 43 Terms Beginning with H 45 Terms Beginning with I 51 Terms Beginning with J 55 Terms Beginning with K 57 Terms Beginning with L 59 Terms Beginning with M 63 Terms Beginning with N 69 Terms Beginning with O 71 Terms Beginning with P 73 Terms Beginning with Q 81 Terms Beginning with R 83 Terms Beginning with S 87 iii Terms Beginning with T 95 Terms Beginning with U 101 Terms Beginning with V 103 Terms Beginning with W 105 Terms Beginning with X 107 Terms Beginning with Y 109 Terms Beginning with Z Ill iv Acknowledgments Richard D. -

Chemical Names and CAS Numbers Final
Chemical Abstract Chemical Formula Chemical Name Service (CAS) Number C3H8O 1‐propanol C4H7BrO2 2‐bromobutyric acid 80‐58‐0 GeH3COOH 2‐germaacetic acid C4H10 2‐methylpropane 75‐28‐5 C3H8O 2‐propanol 67‐63‐0 C6H10O3 4‐acetylbutyric acid 448671 C4H7BrO2 4‐bromobutyric acid 2623‐87‐2 CH3CHO acetaldehyde CH3CONH2 acetamide C8H9NO2 acetaminophen 103‐90‐2 − C2H3O2 acetate ion − CH3COO acetate ion C2H4O2 acetic acid 64‐19‐7 CH3COOH acetic acid (CH3)2CO acetone CH3COCl acetyl chloride C2H2 acetylene 74‐86‐2 HCCH acetylene C9H8O4 acetylsalicylic acid 50‐78‐2 H2C(CH)CN acrylonitrile C3H7NO2 Ala C3H7NO2 alanine 56‐41‐7 NaAlSi3O3 albite AlSb aluminium antimonide 25152‐52‐7 AlAs aluminium arsenide 22831‐42‐1 AlBO2 aluminium borate 61279‐70‐7 AlBO aluminium boron oxide 12041‐48‐4 AlBr3 aluminium bromide 7727‐15‐3 AlBr3•6H2O aluminium bromide hexahydrate 2149397 AlCl4Cs aluminium caesium tetrachloride 17992‐03‐9 AlCl3 aluminium chloride (anhydrous) 7446‐70‐0 AlCl3•6H2O aluminium chloride hexahydrate 7784‐13‐6 AlClO aluminium chloride oxide 13596‐11‐7 AlB2 aluminium diboride 12041‐50‐8 AlF2 aluminium difluoride 13569‐23‐8 AlF2O aluminium difluoride oxide 38344‐66‐0 AlB12 aluminium dodecaboride 12041‐54‐2 Al2F6 aluminium fluoride 17949‐86‐9 AlF3 aluminium fluoride 7784‐18‐1 Al(CHO2)3 aluminium formate 7360‐53‐4 1 of 75 Chemical Abstract Chemical Formula Chemical Name Service (CAS) Number Al(OH)3 aluminium hydroxide 21645‐51‐2 Al2I6 aluminium iodide 18898‐35‐6 AlI3 aluminium iodide 7784‐23‐8 AlBr aluminium monobromide 22359‐97‐3 AlCl aluminium monochloride -

Toxins in Certain Indigenous Kenya Plants"
"TOXINS IN CERTAIN INDIGENOUS KENYA PLANTS" By SAIFUDDIN F. DOSSAJI L_____ A Thesis Submitted in Part Fulfillment for the Degree of Master of Science in the University of Nairobi 1971 This thesis has not been submitted for a degree in any other university. * aifuddin F. Dossaji -iii- CONTENTS Page A B S T R A C T ......................................... vii ACKNOWLEDGEMENTS................................ CHAPTER I ABSTRACT ......................................... 1 INTRODUCTION ..................................... 2 EXPERIMENTAL RESULTS ............................ 6 DISCUSSION....................................... 10 EXPERIMENTAL WORK ............................... 23 M a t e r i a l s ................................ 23 Paper Chromatography ................... 23 Column Chromatography ................... 24 Isolation of Macrozamin (Experimental Procedure) ............. 26 S U M M A R Y ......................................... 29 REFERENCES....................................... 31 BIBLIOGRAPHY 35 -iv- Page CHAPTER II A B S T R A C T ..............................................41 INTRODUCTION ........................................ 42 EXPERIMENTAL RESULTS AND DISCUSSION .............. 46 EXPERIMENTAL W O R K ................................... 49 E x t r a c t i o n ................................... 49 Paper Chromatography ........................ 50 Synthesis of a-amino-S-methylamino- propionic a c i d ............................ 51 CONCLUDING COMMENT .................................. 54 REFERENCES...........................................‘55 -
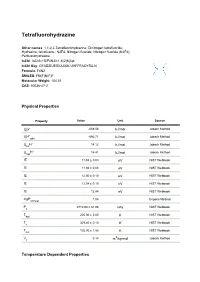
Tetrafluorohydrazine Datasheet
Tetrafluorohydrazine Other names: 1,1,2,2-Tetrafluorohydrazine; Dinitrogen tetrafluoride; Hydrazine, tetrafluoro-; N2F4; Nitrogen fluoride; Nitrogen fluoride (N2F4); Perfluorohydrazine. InChI: InChI=1S/F4N2/c1-5(2)6(3)4 InChI Key: GFADZIUESKAXAK-UHFFFAOYSA-N Formula: F4N2 SMILES: FN(F)N(F)F Molecular Weight: 104.01 CAS: 10036-47-2 Physical Properties Property Value Unit Source ∆ G° -608.56 kJ/mol Joback Method f ∆ H° -692.71 Joback Method f gas kJ/mol ∆ H° 14.12 Joback Method fus kJ/mol ∆ H° 16.41 Joback Method vap kJ/mol IE 11.94 ± 0.03 eV NIST Webbook IE 11.94 ± 0.03 eV NIST Webbook IE 12.00 ± 0.10 eV NIST Webbook IE 12.04 ± 0.10 eV NIST Webbook IE 12.84 eV NIST Webbook logP 1.04 Crippen Method oct/wat P 3710.00 ± 81.06 NIST Webbook c kPa T 200.00 ± 2.00 NIST Webbook boil K T 309.40 ± 0.10 NIST Webbook c K T 105.00 ± 1.50 NIST Webbook fus K V 0.14 3 Joback Method c m /kg-mol Temperature Dependent Properties Property Value Unit Temperature (K) Source C 59.86 J/mol×K 221.36 Joback Method p,gas ∆ H 26.40 200.0 NIST Webbook vap kJ/mol Sources Joback Method: https://en.wikipedia.org/wiki/Joback_method NIST Webbook: http://webbook.nist.gov/cgi/inchi/InChI=1S/F4N2/c1-5(2)6(3)4 Crippen Method: http://pubs.acs.org/doi/abs/10.1021/ci990307l Legend C : Ideal gas heat capacity (J/mol×K). p,gas ∆ G°: Standard Gibbs free energy of formation (kJ/mol). -

Is Exposure to BMAA a Risk Factor for Neurodegenerative Diseases? a Response to a Critical Review of the BMAA Hypothesis
Neurotoxicity Research (2021) 39:81–106 https://doi.org/10.1007/s12640-020-00302-0 S.I. : BMAA Is Exposure to BMAA a Risk Factor for Neurodegenerative Diseases? A Response to a Critical Review of the BMAA Hypothesis Dunlop RA1 · Banack SA1 · Bishop SL2 · Metcalf JS1 · Murch SJ3 · Davis DA4 · Stommel EW5 · Karlsson O6 · Brittebo EB7 · Chatziefthimiou AD8 · Tan VX9 · Guillemin GG9 · Cox PA1 · Mash DC10 · Bradley WG4 Received: 9 September 2020 / Revised: 19 October 2020 / Accepted: 20 October 2020 / Published online: 6 February 2021 © The Author(s) 2021 Abstract In a literature survey, Chernof et al. (2017) dismissed the hypothesis that chronic exposure to β-N-methylamino-L-alanine (BMAA) may be a risk factor for progressive neurodegenerative disease. They question the growing scientifc literature that suggests the following: (1) BMAA exposure causes ALS/PDC among the indigenous Chamorro people of Guam; (2) Gua- manian ALS/PDC shares clinical and neuropathological features with Alzheimer’s disease, Parkinson’s disease, and ALS; (3) one possible mechanism for protein misfolds is misincorporation of BMAA into proteins as a substitute for L-serine; and (4) chronic exposure to BMAA through diet or environmental exposures to cyanobacterial blooms can cause neuro- degenerative disease. We here identify multiple errors in their critique including the following: (1) their review selectively cites the published literature; (2) the authors reported favorably on HILIC methods of BMAA detection while the literature shows signifcant matrix efects and peak coelution in HILIC that may prevent detection and quantifcation of BMAA in cyanobacteria; (3) the authors build alternative arguments to the BMAA hypothesis, rather than explain the published lit- erature which, to date, has been unable to refute the BMAA hypothesis; and (4) the authors erroneously attribute methods to incorrect studies, indicative of a failure to carefully consider all relevant publications. -

Chemical Hygiene Plan
Chemical Hygiene Plan Document Number: EHS-DOC600.02 Chemical Hygiene Plan Table of Contents 1.0 BACKGROUND ......................................................................................................................... 5 1.1 REGULATORY STANDARDS .............................................................................................................. 5 1.2 FLORIDA INTERNATIONAL UNIVERSITY LABORATORY SAFETY STANDARD OPERATING PROCEDURES ............................................................................................................................................... 5 1.3 PLAN DEVELOPMENT, MAINTENANCE, AND REVISION .................................................................. 6 2.0 INTRODUCTION ....................................................................................................................... 7 2.1 PURPOSE ......................................................................................................................................... 7 2.2 SCOPE .............................................................................................................................................. 7 2.3 PROGRAM ADMINISTRATION RESPONSIBILITY AND ACCOUNTABILITY ......................................... 8 2.4 GENERAL LABORATORY SAFETY CONCEPTS .................................................................................. 10 3.0 HEALTH AND SAFETY TRAINING REQUIREMENTS .................................................................... 15 3.1 EMPLOYEE RIGHT-TO-KNOW STANDARD ....................................................................................