A Functional Pre-Screening Platform for Identifying Points of Vulnerability In
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Supplemental Information to Mammadova-Bach Et Al., “Laminin Α1 Orchestrates VEGFA Functions in the Ecosystem of Colorectal Carcinogenesis”
Supplemental information to Mammadova-Bach et al., “Laminin α1 orchestrates VEGFA functions in the ecosystem of colorectal carcinogenesis” Supplemental material and methods Cloning of the villin-LMα1 vector The plasmid pBS-villin-promoter containing the 3.5 Kb of the murine villin promoter, the first non coding exon, 5.5 kb of the first intron and 15 nucleotides of the second villin exon, was generated by S. Robine (Institut Curie, Paris, France). The EcoRI site in the multi cloning site was destroyed by fill in ligation with T4 polymerase according to the manufacturer`s instructions (New England Biolabs, Ozyme, Saint Quentin en Yvelines, France). Site directed mutagenesis (GeneEditor in vitro Site-Directed Mutagenesis system, Promega, Charbonnières-les-Bains, France) was then used to introduce a BsiWI site before the start codon of the villin coding sequence using the 5’ phosphorylated primer: 5’CCTTCTCCTCTAGGCTCGCGTACGATGACGTCGGACTTGCGG3’. A double strand annealed oligonucleotide, 5’GGCCGGACGCGTGAATTCGTCGACGC3’ and 5’GGCCGCGTCGACGAATTCACGC GTCC3’ containing restriction site for MluI, EcoRI and SalI were inserted in the NotI site (present in the multi cloning site), generating the plasmid pBS-villin-promoter-MES. The SV40 polyA region of the pEGFP plasmid (Clontech, Ozyme, Saint Quentin Yvelines, France) was amplified by PCR using primers 5’GGCGCCTCTAGATCATAATCAGCCATA3’ and 5’GGCGCCCTTAAGATACATTGATGAGTT3’ before subcloning into the pGEMTeasy vector (Promega, Charbonnières-les-Bains, France). After EcoRI digestion, the SV40 polyA fragment was purified with the NucleoSpin Extract II kit (Machery-Nagel, Hoerdt, France) and then subcloned into the EcoRI site of the plasmid pBS-villin-promoter-MES. Site directed mutagenesis was used to introduce a BsiWI site (5’ phosphorylated AGCGCAGGGAGCGGCGGCCGTACGATGCGCGGCAGCGGCACG3’) before the initiation codon and a MluI site (5’ phosphorylated 1 CCCGGGCCTGAGCCCTAAACGCGTGCCAGCCTCTGCCCTTGG3’) after the stop codon in the full length cDNA coding for the mouse LMα1 in the pCIS vector (kindly provided by P. -

Gene Symbol Gene Description ACVR1B Activin a Receptor, Type IB
Table S1. Kinase clones included in human kinase cDNA library for yeast two-hybrid screening Gene Symbol Gene Description ACVR1B activin A receptor, type IB ADCK2 aarF domain containing kinase 2 ADCK4 aarF domain containing kinase 4 AGK multiple substrate lipid kinase;MULK AK1 adenylate kinase 1 AK3 adenylate kinase 3 like 1 AK3L1 adenylate kinase 3 ALDH18A1 aldehyde dehydrogenase 18 family, member A1;ALDH18A1 ALK anaplastic lymphoma kinase (Ki-1) ALPK1 alpha-kinase 1 ALPK2 alpha-kinase 2 AMHR2 anti-Mullerian hormone receptor, type II ARAF v-raf murine sarcoma 3611 viral oncogene homolog 1 ARSG arylsulfatase G;ARSG AURKB aurora kinase B AURKC aurora kinase C BCKDK branched chain alpha-ketoacid dehydrogenase kinase BMPR1A bone morphogenetic protein receptor, type IA BMPR2 bone morphogenetic protein receptor, type II (serine/threonine kinase) BRAF v-raf murine sarcoma viral oncogene homolog B1 BRD3 bromodomain containing 3 BRD4 bromodomain containing 4 BTK Bruton agammaglobulinemia tyrosine kinase BUB1 BUB1 budding uninhibited by benzimidazoles 1 homolog (yeast) BUB1B BUB1 budding uninhibited by benzimidazoles 1 homolog beta (yeast) C9orf98 chromosome 9 open reading frame 98;C9orf98 CABC1 chaperone, ABC1 activity of bc1 complex like (S. pombe) CALM1 calmodulin 1 (phosphorylase kinase, delta) CALM2 calmodulin 2 (phosphorylase kinase, delta) CALM3 calmodulin 3 (phosphorylase kinase, delta) CAMK1 calcium/calmodulin-dependent protein kinase I CAMK2A calcium/calmodulin-dependent protein kinase (CaM kinase) II alpha CAMK2B calcium/calmodulin-dependent -

Outlier Kinase Expression by RNA Sequencing As Targets for Precision Therapy
Published OnlineFirst February 5, 2013; DOI: 10.1158/2159-8290.CD-12-0336 RESEARCH ARTICLE Outlier Kinase Expression by RNA Sequencing as Targets for Precision Therapy Vishal Kothari 1 , Iris Wei 2 , Sunita Shankar 1 , 3 , Shanker Kalyana-Sundaram 1 , 3 , 8 , Lidong Wang 2 , Linda W. Ma 1 , Pankaj Vats 1 , Catherine S. Grasso 1 , Dan R. Robinson 1 , 3 , Yi-Mi Wu 1 , 3 , Xuhong Cao 7 , Diane M. Simeone 2 , 4 , 5 , Arul M. Chinnaiyan 1 , 3 , 4 , 6 , 7 , and Chandan Kumar-Sinha 1 , 3 ABSTRACT Protein kinases represent the most effective class of therapeutic targets in cancer; therefore, determination of kinase aberrations is a major focus of cancer genomic studies. Here, we analyzed transcriptome sequencing data from a compendium of 482 cancer and benign samples from 25 different tissue types, and defi ned distinct “outlier kinases” in individual breast and pancreatic cancer samples, based on highest levels of absolute and differential expression. Frequent outlier kinases in breast cancer included therapeutic targets like ERBB2 and FGFR4 , distinct from MET , AKT2 , and PLK2 in pancreatic cancer. Outlier kinases imparted sample-specifi c depend- encies in various cell lines, as tested by siRNA knockdown and/or pharmacologic inhibition. Outlier expression of polo-like kinases was observed in a subset of KRAS -dependent pancreatic cancer cell lines, and conferred increased sensitivity to the pan-PLK inhibitor BI-6727. Our results suggest that outlier kinases represent effective precision therapeutic targets that are readily identifi able through RNA sequencing of tumors. SIGNIFICANCE: Various breast and pancreatic cancer cell lines display sensitivity to knockdown or pharmacologic inhibition of sample-specifi c outlier kinases identifi ed by high-throughput transcrip- tome sequencing. -

The Number of Genes
Table S1. The numbers of KD genes in each KD time The number The number The number The number Cell lines of genes of genes of genes of genes (96h) (120h) (144h) PC3 3980 3822 128 1725 A549 3724 3724 0 0 MCF7 3688 3471 0 1837 HT29 3665 3665 0 0 A375 3826 3826 0 0 HA1E 3801 3801 0 0 VCAP 4134 34 4121 0 HCC515 3522 3522 0 0 Table S2. The predicted results in the PC3 cell line on the LINCS II data id target rank A07563059 ADRB2 48 A12896037 ADRA2C 91 A13021932 YES1 77 PPM1B;PPP1CC;PPP2CA; A13254067 584;1326;297;171;3335 PTPN1;PPP2R5A A16347691 GMNN 2219 PIK3CB;MTOR;PIK3CA;PIK A28467416 18;10;9;13;8 3CG;PIK3CD A28545468 EHMT2;MAOB 14;67 A29520968 HSPB1 1770 A48881734 EZH2 1596 A52922642 CACNA1C 201 A64553394 ADRB2 155 A65730376 DOT1L 3764 A82035391 JUN 378 A82156122 DPP4 771 HRH1;HTR2C;CHRM3;CH A82772293 2756;2354;2808;2367 RM1 A86248581 CDA 1785 A92800748 TEK 459 A93093700 LMNA 1399 K00152668 RARB 105 K01577834 ADORA2A 525 K01674964 HRH1;BLM 31;1314 K02314383 AR 132 K03194791 PDE4D 30 K03390685 MAP2K1 77 K06762493 GMNN;APEX1 1523;2360 K07106112 ERBB4;ERBB2;EGFR 497;60;23 K07310275 AKT1;MTOR;PIK3CA 13;12;1 K07753030 RGS4;BLM 3736;3080 K08109215 BRD2;BRD3;BRD4 1413;2786;3 K08248804 XIAP 88 K08586861 TBXA2R;MBNL1 297;3428 K08832567 GMNN;CA12 2544;50 LMNA;NFKB1;APEX1;EH K08976401 1322;341;3206;123 MT2 K09372874 IMPDH2 232 K09711437 PLA2G2A 59 K10859802 GPR119 214 K11267252 RET;ALK 395;760 K12609457 LMNA 907 K13094524 BRD4 7 K13662825 CDK4;CDK9;CDK5;CDK1 34;58;13;18 K14704277 LMNA;BLM 1697;1238 K14870255 AXL 1696 K15170068 MAN2B1 1756 K15179879 -

14-3-3 Proteins Inactivate DAPK2 by Promoting Its Dimerization And
ARTICLE https://doi.org/10.1038/s42003-021-02518-y OPEN 14-3-3 proteins inactivate DAPK2 by promoting its dimerization and protecting key regulatory phosphosites ✉ ✉ Matej Horvath 1,2, Olivia Petrvalska1,2, Petr Herman 3, Veronika Obsilova 2 & Tomas Obsil 1,2 Death-associated protein kinase 2 (DAPK2) is a CaM-regulated Ser/Thr protein kinase, involved in apoptosis, autophagy, granulocyte differentiation and motility regulation, whose activity is controlled by autoinhibition, autophosphorylation, dimerization and interaction with scaffolding proteins 14-3-3. However, the structural basis of 14-3-3-mediated DAPK2 reg- ulation remains unclear. Here, we structurally and biochemically characterize the full-length 1234567890():,; human DAPK2:14-3-3 complex by combining several biophysical techniques. The results from our X-ray crystallographic analysis revealed that Thr369 phosphorylation at the DAPK2 C terminus creates a high-affinity canonical mode III 14-3-3-binding motif, further enhanced by the diterpene glycoside Fusicoccin A. Moreover, concentration-dependent DAPK2 dimerization is disrupted by Ca2+/CaM binding and stabilized by 14-3-3 binding in solution, thereby protecting the DAPK2 inhibitory autophosphorylation site Ser318 against depho- sphorylation and preventing Ca2+/CaM binding. Overall, our findings provide mechanistic insights into 14-3-3-mediated DAPK2 inhibition and highlight the potential of the DAPK2:14- 3-3 complex as a target for anti‐inflammatory therapies. 1 Department of Physical and Macromolecular Chemistry, Faculty of Science, Charles University, Prague, Czech Republic. 2 Department of Structural Biology of Signaling Proteins, Division BIOCEV, Institute of Physiology of the Czech Academy of Sciences, Vestec, Czech Republic. 3 Institute of Physics, Faculty of ✉ Mathematics and Physics, Charles University, Prague, Czech Republic. -

Novel Functions of Death-Associated Protein Kinases Through Mitogen-Activated Protein Kinase-Related Signals
International Journal of Molecular Sciences Article Novel Functions of Death-Associated Protein Kinases through Mitogen-Activated Protein Kinase-Related Signals Mohamed Elbadawy 1,2,† , Tatsuya Usui 1,*,†, Hideyuki Yamawaki 3 and Kazuaki Sasaki 1 1 Laboratory of Veterinary Pharmacology, Department of Veterinary Medicine, Faculty of Agriculture, Tokyo University of Agriculture and Technology, 3-5-8 Saiwai-cho, Fuchu, Tokyo 183-8509, Japan; [email protected] (M.E.); [email protected] (K.S.) 2 Department of Pharmacology, Faculty of Veterinary Medicine, Benha University, Moshtohor, Elqaliobiya, Toukh 13736, Egypt 3 Laboratory of Veterinary Pharmacology, School of Veterinary Medicine, Kitasato University, Towada, Aomori 034-8628, Japan; [email protected] * Correspondence: [email protected]; Tel./Fax: +81-42-367-5769 † These authors contributed equally to this work. Received: 13 September 2018; Accepted: 1 October 2018; Published: 4 October 2018 Abstract: Death associated protein kinase (DAPK) is a calcium/calmodulin-regulated serine/threonine kinase; its main function is to regulate cell death. DAPK family proteins consist of DAPK1, DAPK2, DAPK3, DAPK-related apoptosis-inducing protein kinases (DRAK)-1 and DRAK-2. In this review, we discuss the roles and regulatory mechanisms of DAPK family members and their relevance to diseases. Furthermore, a special focus is given to several reports describing cross-talks between DAPKs and mitogen-activated protein kinases (MAPK) family members in various pathologies. We also discuss small molecule inhibitors of DAPKs and their potential as therapeutic targets against human diseases. Keywords: MAPK; DAPK; ERK; p38; JNK 1. Introduction: DAPKs, MAPKs Death-associated protein kinase (DAPK) family proteins are closely related, Ca2+/calmodulin (CaM)-regulated serine/threonine kinases, whose members not only possess significant homology in their catalytic domains but also share cell death-associated functions [1,2]. -

Anti-DAPK2 Antibody (ARG54388)
Product datasheet [email protected] ARG54388 Package: 50 μg anti-DAPK2 antibody Store at: -20°C Summary Product Description Rabbit Polyclonal antibody recognizes DAPK2 Tested Reactivity Hu, Ms, Rat Tested Application IHC, WB Specificity This antibody recognizes human, mouse, and rat DAPK2 (approx. 42kDa) and does not cross-react with DAPK. Host Rabbit Clonality Polyclonal Isotype IgG Target Name DAPK2 Antigen Species Human Immunogen Peptide corresponding to aa 356-370 of human DAPK2 (accession no. BAA88063). This sequence is identical to that of mouse. Conjugation Un-conjugated Alternate Names DRP1; DAP-kinase-related protein 1; DRP-1; DAP kinase 2; EC 2.7.11.1; Death-associated protein kinase 2 Application Instructions Application table Application Dilution IHC Assay-dependent WB Assay-dependent Application Note * The dilutions indicate recommended starting dilutions and the optimal dilutions or concentrations should be determined by the scientist. Positive Control A431, Mouse spleen and Rat kidney Calculated Mw 43 kDa Properties Form Liquid Purification Immunoaffinity chroma-tography Buffer PBS (pH 7.4) and 0.02% Sodium azide Preservative 0.02% Sodium azide Storage instruction For continuous use, store undiluted antibody at 2-8°C for up to a week. For long-term storage, aliquot and store at -20°C or below. Storage in frost free freezers is not recommended. Avoid repeated freeze/thaw cycles. Suggest spin the vial prior to opening. The antibody solution should be gently mixed www.arigobio.com 1/3 before use. Note For laboratory research only, not for drug, diagnostic or other use. Bioinformation Database links GeneID: 13143 Mouse GeneID: 23604 Human Swiss-port # Q8VDF3 Mouse Swiss-port # Q9UIK4 Human Gene Symbol DAPK2 Gene Full Name death-associated protein kinase 2 Background Certain serine/threonine protein kinases, such as RIP and DAP kinase, are mediators of apoptosis. -

Novel Methylation Patterns Predict Outcome in Uveal Melanoma
Article Novel Methylation Patterns Predict Outcome in Uveal Melanoma Sarah Tadhg Ferrier 1 and Julia Valdemarin Burnier 1,2,3,* 1 Cancer Research Program, Research Institute of the McGill University Health Centre, Montreal, QC, Canada, H4A 3J1; [email protected] 2 Experimental Pathology Unit, Department of Pathology, McGill University; Montreal, QC, Canada, H3A 0G4 3 Department of Oncology, McGill University; Montreal, QC, Canada, H3A 0G4 * Correspondence: [email protected] Table S1. Differentially methylated genes in the Pathways in Cancer KEGG pathway with a log FC ≥ 1.5. Average Average Log Fold Change Differentially Adjusted P Beta Beta ID Gene Name Species (High vs Low Methylated Probes Value Value, Value, Risk) Low High ABL proto-oncogene 1, non- ABL1 Homo sapiens cg13440206, −1.85238 1.39E−06 0.576589 0.259088 receptor tyrosine kinase(ABL1) cg02915920 −1.84042 8.03E−06 0.482846 0.192714 cg21195763 1.685721 3.13E−19 0.573548 0.83358 ADCY2 adenylate cyclase 2(ADCY2) Homo sapiens cg14116052 2.454448 4.3E−24 0.513149 0.885217 ADCY6 adenylate cyclase 6(ADCY6) Homo sapiens cg25196508 3.480923 2.9E−25 0.188362 0.792499 AKT serine/threonine kinase AKT1 Homo sapiens cg14116052 2.454448 4.3E−24 0.513149 0.885217 1(AKT1) bone morphogenetic protein BMP4 Homo sapiens cg08046044 1.527233 3.98E−06 0.049923 0.209543 4(BMP4) cg01873886 1.789942 2.55E−05 0.026254 0.1723 Life 2020, 10, x; doi: FOR PEER REVIEW www.mdpi.com/journal/life Life 2020, 10, x FOR PEER REVIEW 2 of 22 cyclin dependent kinase inhibitor CDKN1B Homo sapiens cg06197769 -

Inhibition of ERK 1/2 Kinases Prevents Tendon Matrix Breakdown Ulrich Blache1,2,3, Stefania L
www.nature.com/scientificreports OPEN Inhibition of ERK 1/2 kinases prevents tendon matrix breakdown Ulrich Blache1,2,3, Stefania L. Wunderli1,2,3, Amro A. Hussien1,2, Tino Stauber1,2, Gabriel Flückiger1,2, Maja Bollhalder1,2, Barbara Niederöst1,2, Sandro F. Fucentese1 & Jess G. Snedeker1,2* Tendon extracellular matrix (ECM) mechanical unloading results in tissue degradation and breakdown, with niche-dependent cellular stress directing proteolytic degradation of tendon. Here, we show that the extracellular-signal regulated kinase (ERK) pathway is central in tendon degradation of load-deprived tissue explants. We show that ERK 1/2 are highly phosphorylated in mechanically unloaded tendon fascicles in a vascular niche-dependent manner. Pharmacological inhibition of ERK 1/2 abolishes the induction of ECM catabolic gene expression (MMPs) and fully prevents loss of mechanical properties. Moreover, ERK 1/2 inhibition in unloaded tendon fascicles suppresses features of pathological tissue remodeling such as collagen type 3 matrix switch and the induction of the pro-fbrotic cytokine interleukin 11. This work demonstrates ERK signaling as a central checkpoint to trigger tendon matrix degradation and remodeling using load-deprived tissue explants. Tendon is a musculoskeletal tissue that transmits muscle force to bone. To accomplish its biomechanical function, tendon tissues adopt a specialized extracellular matrix (ECM) structure1. Te load-bearing tendon compart- ment consists of highly aligned collagen-rich fascicles that are interspersed with tendon stromal cells. Tendon is a mechanosensitive tissue whereby physiological mechanical loading is vital for maintaining tendon archi- tecture and homeostasis2. Mechanical unloading of the tissue, for instance following tendon rupture or more localized micro trauma, leads to proteolytic breakdown of the tissue with severe deterioration of both structural and mechanical properties3–5. -
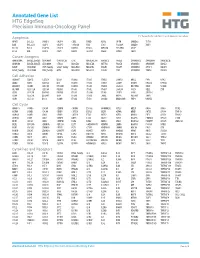
Annotated Gene List HTG Edgeseq Precision Immuno-Oncology Panel
Annotated Gene List HTG EdgeSeq Precision Immuno-Oncology Panel For Research Use Only. Not for use in diagnostic procedures. Apoptosis APAF1 BCL2L1 CARD11 CASP4 CD5L FADD KSR2 OPTN SAMD12 TCF19 BAX BCL2L11 CASP1 CASP5 CORO1A FAS LRG1 PLA2G6 SAMD9 XAF1 BCL10 BCL6 CASP10 CASP8 DAPK2 FASLG MECOM PYCARD SPOP BCL2 BID CASP3 CAV1 DAPL1 GLIPR1 MELK RIPK2 TBK1 Cancer Antigens ANKRD30A BAGE2_BAGE3 CEACAM6 CTAG1A_1B LIPE MAGEA3_A6 MAGEC2 PAGE3 SPANXACD SPANXN4 XAGE1B_1E ARMCX6 BAGE4_BAGE5 CEACAM8 CTAG2 MAGEA1 MAGEA4 MTFR2 PAGE4 SPANXB1 SPANXN5 XAGE2 BAGE CEACAM1 CT45_family GAGE_family MAGEA10 MAGEB2 PAGE1 PAGE5 SPANXN1 SYCP1 XAGE3 BAGE_family CEACAM5 CT47_family HPN MAGEA12 MAGEC1 PAGE2 PBK SPANXN3 TEX14 XAGE5 Cell Adhesion ADAM17 CDH15 CLEC5A DSG3 ICAM2 ITGA5 ITGB2 LAMC3 MBL2 PVR UPK2 ADD2 CDH5 CLEC6A DST ICAM3 ITGA6 ITGB3 LAMP1 MTDH RRAS2 UPK3A ADGRE5 CLDN3 CLEC7A EPCAM ICAM4 ITGAE ITGB4 LGALS1 NECTIN2 SELE VCAM1 ALCAM CLEC12A CLEC9A FBLN1 ITGA1 ITGAL ITGB7 LGALS3 OCLN SELL ZYX CD63 CLEC2B DIAPH3 FXYD5 ITGA2 ITGAM ITLN2 LYVE1 OLR1 SELPLG CD99 CLEC4A DLGAP5 IBSP ITGA3 ITGAX JAML M6PR PECAM1 THY1 CDH1 CLEC4C DSC3 ICAM1 ITGA4 ITGB1 L1CAM MADCAM1 PKP1 UNC5D Cell Cycle ANAPC1 CCND3 CDCA5 CENPH CNNM1 ESCO2 HORMAD2 KIF2C MELK ORC6 SKA3 TPX2 ASPM CCNE1 CDCA8 CENPI CNTLN ESPL1 IKZF1 KIF4A MND1 PATZ1 SP100 TRIP13 AURKA CCNE2 CDK1 CENPL CNTLN ETS1 IKZF2 KIF5C MYBL2 PIF1 SP110 TROAP AURKB CCNF CDK4 CENPU DBF4 ETS2 IKZF3 KIFC1 NCAPG PIMREG SPC24 TUBB BEX1 CDC20 CDK6 CENPW E2F2 EZH2 IKZF4 KNL1 NCAPG2 PKMYT1 SPC25 ZWILCH BEX2 CDC25A CDKN1A CEP250 E2F7 GADD45GIP1 -

PRODUCTS and SERVICES Target List
PRODUCTS AND SERVICES Target list Kinase Products P.1-11 Kinase Products Biochemical Assays P.12 "QuickScout Screening Assist™ Kits" Kinase Protein Assay Kits P.13 "QuickScout Custom Profiling & Panel Profiling Series" Targets P.14 "QuickScout Custom Profiling Series" Preincubation Targets Cell-Based Assays P.15 NanoBRET™ TE Intracellular Kinase Cell-Based Assay Service Targets P.16 Tyrosine Kinase Ba/F3 Cell-Based Assay Service Targets P.17 Kinase HEK293 Cell-Based Assay Service ~ClariCELL™ ~ Targets P.18 Detection of Protein-Protein Interactions ~ProbeX™~ Stable Cell Lines Crystallization Services P.19 FastLane™ Structures ~Premium~ P.20-21 FastLane™ Structures ~Standard~ Kinase Products For details of products, please see "PRODUCTS AND SERVICES" on page 1~3. Tyrosine Kinases Note: Please contact us for availability or further information. Information may be changed without notice. Expression Protein Kinase Tag Carna Product Name Catalog No. Construct Sequence Accession Number Tag Location System HIS ABL(ABL1) 08-001 Full-length 2-1130 NP_005148.2 N-terminal His Insect (sf21) ABL(ABL1) BTN BTN-ABL(ABL1) 08-401-20N Full-length 2-1130 NP_005148.2 N-terminal DYKDDDDK Insect (sf21) ABL(ABL1) [E255K] HIS ABL(ABL1)[E255K] 08-094 Full-length 2-1130 NP_005148.2 N-terminal His Insect (sf21) HIS ABL(ABL1)[T315I] 08-093 Full-length 2-1130 NP_005148.2 N-terminal His Insect (sf21) ABL(ABL1) [T315I] BTN BTN-ABL(ABL1)[T315I] 08-493-20N Full-length 2-1130 NP_005148.2 N-terminal DYKDDDDK Insect (sf21) ACK(TNK2) GST ACK(TNK2) 08-196 Catalytic domain -

Gene Section Review
Atlas of Genetics and Cytogenetics in Oncology and Haematology OPEN ACCESS JOURNAL INIST-CNRS Gene Section Review DAPK2 (death-associated protein kinase 2) Mafalda Pinto, Valdemar Máximo IPATIMUP Institute of Molecular Pathology and Immunology of the University of Porto, (MP, VM); I3S Institute for Innovation and Health Research, University of Porto (MP, VM); Department of Pathology and Oncology, Medical Faculty of the University of Porto, Porto, Portugal (VM) [email protected]; [email protected] Published in Atlas Database: April 2016 Online updated version : http://AtlasGeneticsOncology.org/Genes/DAPK2ID40263ch15q22.html Printable original version : http://documents.irevues.inist.fr/bitstream/handle/2042/66944/04-2016-DAPK2ID40263ch15q22.pdf DOI: 10.4267/2042/66944 This work is licensed under a Creative Commons Attribution-Noncommercial-No Derivative Works 2.0 France Licence. © 2016 Atlas of Genetics and Cytogenetics in Oncology and Haematology Location (base pair): Chromosome 15:63,907,036- Abstract 64,072,033 reverse strand. GRCh38:CM000677.2 (Ensembl.org) Short communication on DAPK2, with data on DNA and on the protein encoded. DNA/RNA Keywords DAPK2; DRP1; DRP-1; calcium/calmodulin; DAPK2 is a gene that codes for a protein that serine/threonine; kinase; apoptosis belongs to the serine/threonine protein kinase family. Identity Transcription Other names: DRP1, DRP-1 DPAK2 has 13 transcripts (3 coding), 75 orthologues and 11 paralogues (MYLK4, MYLK3, STK17A, HGNC (Hugo): DAPK2 DAPK3, DAPK1, OBSCN, SPEG, TTN, MYLK, Location: 15q22.31 STK17B, MYKL2) (ENSG00000035664). Blue highlighting indicates alternating exons; Red highlighting indicates amino acids encoded across a splice junction. Atlas Genet Cytogenet Oncol Haematol. 2016; 20(12) 587 DAPK2 (death-associated protein kinase 2) Pinto M, Máximo V Schematic diagram of DAPK2 protein structure.