Changes in the Abundance of Danish Orchids Over the Past 30
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Pdf of JHOS January 2012
JJoouurrnnaall of the HHAARRDDYY OORRCCHHIIDD SSOOCCIIEETTYY Vol. 9 No. 1 (63) January 2012 JOURNAL of the HARDY ORCHID SOCIETY Vol. 9 No. 1 (63) January 2012 The Hardy Orchid Society Our aim is to promote interest in the study of Native European Orchids and those from similar temperate climates throughout the world. We cover such varied aspects as field study, cultivation and propagation, photography, taxonomy and systematics, and practical conservation. We welcome articles relating to any of these subjects, which will be considered for publication by the editorial committee. Please send your submissions to the Editor, and please structure your text according to the “Advice to Authors” (see website www.hardyorchidsociety.org.uk , January 2004 Journal, Members’ Handbook or contact the Editor). Views expressed in journal arti - cles are those of their author(s) and may not reflect those of HOS. The Hardy Orchid Society Committee President: Prof. Richard Bateman, Jodrell Laboratory, Royal Botanic Gardens Kew, Richmond, Surrey, TW9 3DS Chairman: Celia Wright, The Windmill, Vennington, Westbury, Shrewsbury, Shropshire, SY5 9RG [email protected] Vice-Chairman: David Hughes, Linmoor Cottage, Highwood, Ringwood, Hants., BH24 3LE [email protected] Secretary: Alan Leck, 61 Fraser Close, Deeping St. James, Peterborough, PE6 8QL [email protected] Treasurer: John Wallington, 17, Springbank, Eversley Park Road, London, N21 1JH [email protected] Membership Secretary: Moira Tarrant, Bumbys, Fox Road, Mashbury, -

The Genomic Impact of Mycoheterotrophy in Orchids
fpls-12-632033 June 8, 2021 Time: 12:45 # 1 ORIGINAL RESEARCH published: 09 June 2021 doi: 10.3389/fpls.2021.632033 The Genomic Impact of Mycoheterotrophy in Orchids Marcin J ˛akalski1, Julita Minasiewicz1, José Caius2,3, Michał May1, Marc-André Selosse1,4† and Etienne Delannoy2,3*† 1 Department of Plant Taxonomy and Nature Conservation, Faculty of Biology, University of Gdansk,´ Gdansk,´ Poland, 2 Institute of Plant Sciences Paris-Saclay, Université Paris-Saclay, CNRS, INRAE, Univ Evry, Orsay, France, 3 Université de Paris, CNRS, INRAE, Institute of Plant Sciences Paris-Saclay, Orsay, France, 4 Sorbonne Université, CNRS, EPHE, Muséum National d’Histoire Naturelle, Institut de Systématique, Evolution, Biodiversité, Paris, France Mycoheterotrophic plants have lost the ability to photosynthesize and obtain essential mineral and organic nutrients from associated soil fungi. Despite involving radical changes in life history traits and ecological requirements, the transition from autotrophy Edited by: Susann Wicke, to mycoheterotrophy has occurred independently in many major lineages of land Humboldt University of Berlin, plants, most frequently in Orchidaceae. Yet the molecular mechanisms underlying this Germany shift are still poorly understood. A comparison of the transcriptomes of Epipogium Reviewed by: Maria D. Logacheva, aphyllum and Neottia nidus-avis, two completely mycoheterotrophic orchids, to other Skolkovo Institute of Science autotrophic and mycoheterotrophic orchids showed the unexpected retention of several and Technology, Russia genes associated with photosynthetic activities. In addition to these selected retentions, Sean W. Graham, University of British Columbia, the analysis of their expression profiles showed that many orthologs had inverted Canada underground/aboveground expression ratios compared to autotrophic species. Fatty Craig Barrett, West Virginia University, United States acid and amino acid biosynthesis as well as primary cell wall metabolism were among *Correspondence: the pathways most impacted by this expression reprogramming. -

In Vitro Germination, Protocorm Formation, and Plantlet Development of Orchis Coriophora (Orchidaceae), a Naturally Growing Orchid Species in Turkey
Turkish Journal of Botany Turk J Bot (2013) 37: 336-342 http://journals.tubitak.gov.tr/botany/ © TÜBİTAK Research Article doi:10.3906/bot-1205-28 In vitro germination, protocorm formation, and plantlet development of Orchis coriophora (Orchidaceae), a naturally growing orchid species in Turkey Ersan BEKTAŞ*, Mustafa CÜCE, Atalay SÖKMEN Department of Biology, Faculty of Sciences, Karadeniz Technical University, 61080 Trabzon, Turkey Received: 23.05.2012 Accepted: 22.11.2012 Published Online: 15.03.2013 Printed: 15.04.2013 Abstract: Some species belonging to the genus Orchis Tourn. ex L. (Orchidaceae) are of great economic importance as their tubers or corms are used to produce a hot beverage called salep. Nevertheless, these plants are not cultivated but are rather collected from nature, and due to careless collection many have already been listed as endangered plants. In order to assess the possibility of in vitro propagation, an orchid, Orchis coriophora L., was selected as a model plant, and the effects of basal media and plant growth regulators on in vitro seed germination, protocorm development, and plantlet formation were studied. Mature seeds were cultured in 4 different basal media, each supplemented with various concentrations and/or combinations of auxins and cytokinins/cytokinin-like substances. The highest germination rate (44.2%) was observed in Orchimax medium including activated charcoal plus 1 mg/L indole-3-acetic acid. Protocorms developed plantlets in all the tested media. Orchimax medium including activated charcoal and supplemented with 0.25 mg/L 6-benzyladenine was found to be the most suitable medium for the formation of plantlets from protocorms. -
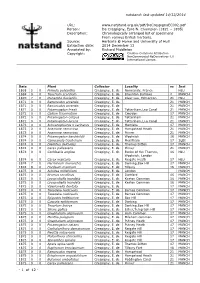
Date Plant Collector Locality Vc Inst 1868 5 0 Primula Polyantha Crespigny, E
natstand: last updated 14/12/2014 URL: www.natstand.org.uk/pdf/DeCrespignyEC002.pdf Person: De Crespigny, Eyre N. Champion (1821 – 1895) Description: Chronologically arranged list of specimens From various British herbaris. Source: Herbaria @ Home and University of Hull Extraction date: 2014 December 13 Annotated by: Richard Middleton Copyright: Creative Commons Attribution- NonCommercial-NoDerivatives 4.0 International License. Date Plant Collector Locality vc Inst 1868 5 0 Primula polyantha Crespigny, E. de Normandy, France HLU 1869 0 0 Teucrium scordium Crespigny, E. de Braunton Burrows 4 MANCH 1870 7 0 Oenanthe fluviatilis Crespigny, E. de River Lee, Edmonton 21 HLU 1871 0 0 Ranunculus arvensis Crespigny, E. de 21 MANCH 1871 0 0 Ranunculus arvensis Crespigny, E. de 21 MANCH 1871 0 0 Potamogeton friesii Crespigny, E. de Tottenham,Lea Canal 21 MANCH 1872 0 0 Galium tricornutum Crespigny, E. de Croydon 17 MANCH 1872 0 0 Potamogeton crispus Crespigny, E. de Tottenham 21 MANCH 1872 0 0 Potamogeton lucens Crespigny, E. de Tottenham,Lea Canal 21 MANCH 1873 0 0 Schoenoplectus x carinatus Crespigny, E. de Mortlake 17 MANCH 1873 0 0 Anemone nemorosa Crespigny, E. de Hampstead Heath 21 MANCH 1873 0 0 Anemone nemorosa Crespigny, E. de Pinner 21 MANCH 1874 0 0 Potamogeton berchtoldii Crespigny, E. de Woolwich 16 MANCH 1874 0 0 Campanula trachelium Crespigny, E. de Merstham 17 SLBI 1874 0 0 Dianthus deltoides Crespigny, E. de Thames Ditton 17 MANCH 1874 0 0 Carex pallescens Crespigny, E. de Pinner 21 MANCH 1874 0 0 Cochlearia anglica Crespigny, E. de Banks of the Thames, 16 HLU Woolwich, London 1874 6 0 Carex vesicaria Crespigny, E. -

Review Article Conservation Status of the Family Orchidaceae in Spain Based on European, National, and Regional Catalogues of Protected Species
Hind ile Scientific Volume 2018, Article ID 7958689, 18 pages https://doi.org/10.1155/2018/7958689 Hindawi Review Article Conservation Status of the Family Orchidaceae in Spain Based on European, National, and Regional Catalogues of Protected Species Daniel de la Torre Llorente© Biotechnology-Plant Biology Department, Higher Technical School of Agronomic, Food and Biosystems Engineering, Universidad Politecnica de Madrid, 28140 Madrid, Spain Correspondence should be addressed to Daniel de la Torre Llorente; [email protected] Received 22 June 2017; Accepted 28 December 2017; Published 30 January 2018 Academic Editor: Antonio Amorim Copyright © 2018 Daniel de la Torre Llorente. Tis is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Tis report reviews the European, National, and Regional catalogues of protected species, focusing specifcally on the Orchidaceae family to determine which species seem to be well-protected and where they are protected. Moreover, this examination highlights which species appear to be underprotected and therefore need to be included in some catalogues of protection or be catalogued under some category of protection. Te national and regional catalogues that should be implemented are shown, as well as what species should be included within them. Tis report should be a helpful guideline for environmental policies about orchids conservation in Spain, at least at the regional and national level. Around 76% of the Spanish orchid fora are listed with any fgure of protection or included in any red list, either nationally (about 12-17%) or regionally (72%). -

Actes Du 15E Colloque Sur Les Orchidées De La Société Française D’Orchidophilie
Cah. Soc. Fr. Orch., n° 7 (2010) – Actes 15e colloque de la Société Française d’Orchidophilie, Montpellier Actes du 15e colloque sur les Orchidées de la Société Française d’Orchidophilie du 30 mai au 1er juin 2009 Montpellier, Le Corum Comité d’organisation : Daniel Prat, Francis Dabonneville, Philippe Feldmann, Michel Nicole, Aline Raynal-Roques, Marc-Andre Selosse, Bertrand Schatz Coordinateurs des Actes Daniel Prat & Bertrand Schatz Affiche du Colloque : Conception : Francis Dabonneville Photographies de Francis Dabonneville & Bertrand Schatz Cahiers de la Société Française d’Orchidophilie, N° 7, Actes du 15e Colloque sur les orchidées de la Société Française d’Orchidophilie. ISSN 0750-0386 © SFO, Paris, 2010 Certificat d’inscription à la commission paritaire N° 55828 ISBN 978-2-905734-17-4 Actes du 15e colloque sur les Orchidées de la Société Française d’Orchidophilie, D. Prat et B. Schatz, Coordinateurs, SFO, Paris, 2010, 236 p. Société Française d’Orchidophilie 17 Quai de la Seine, 75019 Paris Cah. Soc. Fr. Orch., n° 7 (2010) – Actes 15e colloque de la Société Française d’Orchidophilie, Montpellier Préface Ce 15e colloque marque le 40e anniversaire de notre société, celle-ci ayant vu le jour en 1969. Notre dernier colloque se tenait il y a 10 ans à Paris en 1999, 10 ans c’est long, 10 ans c’est très loin. Il fallait que la SFO renoue avec cette traditionnelle organisation de colloques, manifestation qui a contribué à lui accorder la place prépondérante qu’elle occupe au sein des orchidophiles français et de la communauté scientifique. C’est chose faite aujourd’hui. Nombreux sont les thèmes qui font l’objet de communications par des intervenants dont les compétences dans le domaine de l’orchidologie ne sont plus à prouver. -

Watsonia 14 (1982), 79-100
Walsonia , 14, 79-100 (\982) 79 Book Reviews Flowers of Greece and the Balkans. A field guide. Oleg Polunin. Pp. xv+592 including 62 pages of line drawings and 21 maps, with 80 colour plates. Oxford University Press, Oxford. 1980. Price £40.00 (ISBN 0-19- 217-6269). This latest of Oleg Polunin's Field guides to the European flora presents a concise, but by no means superficial, picture of the flowering plants and conifers of the Balkan peninsula. The area that is covers takes in the whole of Greece (including the East Aegean Islands, excluded from Flora Europaea), Turkey-in-Europe, Albania, Bulgaria, Jugoslavia as far north as the River Sava, and the small portion of Romania that lies south and east of the River Danube. The author has condensed his account of the rich and varied flora of the region, together with the associated mass of published material, into a form that is attractive, comprehensible and useful to the amateur botanist. A book of this type has been badly needed, as the available floristic texts on the Balkans tend to be over 50 years old, scarce, extremely expensive and written in a foreign language, often Latin. However, the most significant attribute of this book is not that it gives access to diffuse and obscure information, but that it provides a radical alternative to popular botanical accounts of Greece which emphasize the lowland spring flora, notably the orchids and other petaloid monocots. Based on the author's many years of botanical experience and extensive travel in the region, Flowers of Greece and the Balkans demonstrates the wide range of flora and vegetation that the amateur botanist can expect to see in the Balkan peninsula. -

Die Gattung Epipactis Und Ihre Systematische Stellung Innnerhalb Der Unterfamilie Neottioideae, Im Lichte Enhvickiungsgeschichtli- Eher Untersuchungen
Jber. natnrwiss. Ver Wuppertal 51 43 - 100 Wuppertal, 15.9.1998 Die Gattung Epipactis und ihre systematische Stellung innnerhalb der Unterfamilie Neottioideae, im Lichte enhvickiungsgeschichtli- eher Untersuchungen. Kar1 Robatsch Mit Zeichnungen von L. FREIDINGER und C. A. MRKVICKA Zusammenfassung: Die systematische Stellung der Gattung Epipactis in der Subtribus Cephalantherinae wie auch die Stel- lung dieser Subtribus innerhalb der Unterfamilie Neottioideae wird an Beispielen enhvickungsgeschicht- licher Untersuchungen diskutiert. Nach den neuesten molekularen Daten, die aus DNA-Sequenzanalysen gewonnen wurden, ist ein Stammbaum erstellt worden, in dem die Neottioideae in die "epidendroids" eingereiht wurden. Das steht im Widerspruch zu dem in unserer Arbeit praktizierten Klassifikations- System, das in "Die Orcliideen" R. SCHLECHTER in der Bearbeitung von F. BMEGER und K. SENGHAS venvendet wird. Die Ableitung einer Orchideenblüte aus dem Liliiflorae-Erbe ermögliclit eine Differentialdiagnose zwi- schen den Orchidaceae und den Apostasiaceae. Die Apostasiaceae, die viele Autoren als Unterfamilie Apostasioideae zu den Orchidaceae stellen, werden durch vergleichende Blütenanalysen von dieser Familie abgetrennt. Der Entwickiungstendenz des Gynoeceums der Orchideen, durch die es zum Auf- bau eines Rostellums mit seinen Organen kommt, steht die Reduktionstendenz des Gynoeceums der Apostasiaceae, durch die es zu einer Verminderung des ursprünglich trimeren Stigmas kommt, gegen- über. Die Autogamie der Gattung Epipactis (Sektion Epipactis) -

Forma Nov.: a New Peloric Orchid from Ibaraki Prefecture, Japan
The Japanese Society for Plant Systematics ISSN 1346-7565 Acta Phytotax. Geobot. 65 (3): 127–139 (2014) Cephalanthera falcata f. conformis (Orchidaceae) forma nov.: A New Peloric Orchid from Ibaraki Prefecture, Japan 1,* 1 1 HirosHi Hayakawa , CHiHiro Hayakawa , yosHinobu kusumoto , tomoko 1 1 2 3,4 nisHida , Hiroaki ikeda , tatsuya Fukuda and Jun yokoyama 1National Institute for Agro-Environmental Sciences, 3-1-3 Kannondai, Tsukuba, Ibaraki 305-8604, Japan. *[email protected] (author for correspondences); 2Faculty of Agriculture, Kochi University, Nan- koku, Kochi 783-8502, Japan; 3Faculty of Science, Yamagata University, Kojirakawa, Yamagata 990-8560, Japan; 4Institute for Regional Innovation, Yamagata University, Kaminoyama, Yamagata 999-3101, Japan We recognize a new peloric form of the orchid Cephalanthera falcata (Thunb.) Blume f. conformis Hi- ros. Hayak. et J. Yokoy., which occurs in the vicinity of the Tsukuba mountain range in Ibaraki Prefec- ture, Japan. This peloric form is sympatric with C. falcata f. falcata. The peloric flowers have a petal-like lip. The flowers are radially symmetrical and the perianth parts spread weakly compared to normal flow- ers. The stigmas are positioned at the column apex, thus neighboring stigmas and the lower parts of the pollinia are conglutinated. We observed similar vegetative traits among C. falcata f. falcata, C. falcata f. albescens S. Kobayashi, and C. falcata f. conformis. The floral morphology in C. falcata f. conformis resembles that of C. nanchuanica (S.C. Chen) X.H. Jin et X.G. Xiang (i.e., similar petal-like lip and stig- ma position at the column apex; syn. Tangtsinia nanchuanica S.C. -

Phytogeographical Analysis and Ecological Factors of the Distribution of Orchidaceae Taxa in the Western Carpathians (Local Study)
plants Article Phytogeographical Analysis and Ecological Factors of the Distribution of Orchidaceae Taxa in the Western Carpathians (Local study) Lukáš Wittlinger and Lucia Petrikoviˇcová * Department of Geography and Regional Development, Faculty of Natural Sciences, Constantine the Philosopher University in Nitra, 94974 Nitra, Slovakia; [email protected] * Correspondence: [email protected]; Tel.: +421-907-3441-04 Abstract: In the years 2018–2020, we carried out large-scale mapping in the Western Carpathians with a focus on determining the biodiversity of taxa of the family Orchidaceae using field biogeographical research. We evaluated the research using phytogeographic analysis with an emphasis on selected ecological environmental factors (substrate: ecological land unit value, soil reaction (pH), terrain: slope (◦), flow and hydrogeological productivity (m2.s−1) and average annual amounts of global radiation (kWh.m–2). A total of 19 species were found in the area, of which the majority were Cephalenthera longifolia, Cephalenthera damasonium and Anacamptis morio. Rare findings included Epipactis muelleri, Epipactis leptochila and Limodorum abortivum. We determined the ecological demands of the abiotic environment of individual species by means of a functional analysis of communities. The research confirmed that most of the orchids that were studied occurred in acidified, calcified and basophil locations. From the location of the distribution of individual populations, it is clear that they are generally arranged compactly and occasionally scattered, which results in ecological and environmental diversity. During the research, we identified 129 localities with the occurrence of Citation: Wittlinger, L.; Petrikoviˇcová, L. Phytogeographical Analysis and 19 species and subspecies of orchids. We identify the main factors that threaten them and propose Ecological Factors of the Distribution specific measures to protect vulnerable populations. -

HAUSTORIUM 76 1 HAUSTORIUM Parasitic Plants Newsletter ISSN 1944-6969 Official Organ of the International Parasitic Plant Society (
HAUSTORIUM 76 1 HAUSTORIUM Parasitic Plants Newsletter ISSN 1944-6969 Official Organ of the International Parasitic Plant Society (http://www.parasiticplants.org/) July 2019 Number 76 CONTENTS MESSAGE FROM THE IPPS PRESIDENT (Julie Scholes)………………………………………………..………2 MEETING REPORTS 15th World Congress on Parasitic Plants, 30 June – 5 July 2019, Amsterdam, the Netherlands.………….……..2 MISTLETOE (VISCUM ALBUM) AND ITS HOSTS IN BRITAIN (Brian Spooner)……………………………10 PHELIPANCHE AEGYPTIACA IN WESTERN IRAN (Alireza Taab)……………………………………………12 NEW AND CURRENT PROJECTS Delivering high-yielding, disease-resistant finger millet to farmers…………………………………………….…..13 N2AFRICA – new Striga project – update……………………………………………………………………….…...14 Striga asiatica Madagascar fieldwork summary 2019……………………………………………………………......14 Pea (Pisum sativum) breeding for disease and pest resistance ………………………………………………….......15 REQUEST FOR SEEDS OF OROBANCHE CRENATA (Gianniantonio Domina)…………………………...…..15 PRESS REPORTS Metabolite stimulates a crop while suppressing a weed………………………………………………………….…..16 Dodder plant poses threat to trees and crops (in Kenya)………………………………………………………...….17 PhD OPPORTUNITY AT NRI (Jonne Rodenburg)…………………………………………………………………18 THESIS Sarah Huet. An overview of Phelipanche ramosa seeds: sensitivity to germination stimulants and microbiome profile. …………………………………………………………………………………………………………………..18 BOOK REVIEW Strigolactones – Biology and Applications. Ed. by Hinanit Koltai and Cristina Prandi. (Koichi Yoneyama) …………………………………………………………………………………………………………………………....19 -

Structuration Écologique Et Évolutive Des Symbioses Mycorhiziennes Des
Universit´ede La R´eunion { Ecole´ doctorale Sciences Technologie Sant´e{ Facult´edes Sciences et des Technologies Structuration ´ecologique et ´evolutive des symbioses mycorhiziennes des orchid´ees tropicales Th`ese de doctorat pr´esent´eeet soutenue publiquement le 19 novembre 2010 pour l'obtention du grade de Docteur de l'Universit´ede la R´eunion (sp´ecialit´eBiologie des Populations et Ecologie)´ par Florent Martos Composition du jury Pr´esidente: Mme Pascale Besse, Professeur `al'Universit´ede La R´eunion Rapporteurs : Mme Marie-Louise Cariou, Directrice de recherche au CNRS de Gif-sur-Yvette M. Raymond Tremblay, Professeur `al'Universit´ede Porto Rico Examinatrice : Mme Pascale Besse, Professeur `al'Universit´ede La R´eunion Directeurs : M. Thierry Pailler, Ma^ıtre de conf´erences HDR `al'Universit´ede La R´eunion M. Marc-Andr´e Selosse, Professeur `al'Universit´ede Montpellier Laboratoire d'Ecologie´ Marine Institut de Recherche pour le D´eveloppement 2 R´esum´e Les plantes n'exploitent pas seules les nutriments du sol, mais d´ependent de champignons avec lesquels elles forment des symbioses mycorhiziennes dans leurs racines. C'est en particulier vrai pour les 25 000 esp`ecesd'orchid´eesactuelles qui d´ependent toutes de champignons mycorhiziens pour accomplir leur cycle de vie. Elles produisent des graines microscopiques qui n'ont pas les ressources nutritives pour germer, mais qui d´ependent de la pr´esencede partenaires ad´equatspour nourrir l'embryon (h´et´erotrophie)jusqu’`al'apparition des feuilles (autotrophie). Les myco- rhiziens restent pr´esents dans les racines des adultes o`uils contribuent `ala nutrition, ce qui permet d'´etudierplus facilement la diversit´edes symbiotes `al'aide des ou- tils g´en´etiques.Conscients des biais des ´etudesen faveur des r´egionstemp´er´ees, nous avons ´etudi´ela diversit´edes mycorhiziens d'orchid´eestropicales `aLa R´eunion.