Assessing European Forest Biodiversity: a Multi-Dimensional Approach
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

PRIMARIE 2012 25 NOVEMBREE DALLA 8 ALLE 20** ABBADIA SAN SALVATORE Via Adua, 9 * ASCIANO Saletta Comunale, Via Mameli (Sezioni Elettorali N
PRIMARIE 2012 25 NOVEMBREE DALLA 8 ALLE 20** ABBADIA SAN SALVATORE Via Adua, 9 * ASCIANO Saletta comunale, Via Mameli (sezioni elettorali n. 1, 2, 3 e 6)* Centro Sociale "Asciano Scalo", Via Martiri della Libertà 53 (sezione elettorali n. 4 e 5) Palestra, Viale Toscana, Arbia (sezioni elettorali n. 7 e 8) BUONCONVENTO Casa del Popolo* CASOLE D'ELSA Casole, Sede Pd, via Casolani (sezioni elettorali n. 1 e 2)* Pievescola, Centro polivante* Monteguidi, circolo Arci* Mensano, saletta casolani* Cavallano, saletta centro civico CASTELLINA IN CHIANTI Circolo Sangallo, Via Trento e Trieste, 58* CASTELNUOVO BERARDENGA Castelnuovo paese, Società Filarmonica, Piazza della Citerna 4 (sezioni elettorali n. 1, 2 e 3)* Casetta - Sede Pd, Via Peruzzi, 13 (sezione elettorale n. 4) Pianella - Piscina Comunale (sezioni elettorale 5 e 6) VAGLIAGLI - Sala polisportiva, ex scuola, Via Senese (sezione elettorale n. 7) Quercegrossa - Sede Misericordia, Via Del Chianti Classico, 5 (sezione elettorale 8) CASTIGLIONE D'ORCIA Castiglion d'Orcia, Sede Proloco, Viale Marconi, 13 (sezione elettorale n. 1 e 4)* Campiglia, Centro Civico Via Fiume (sezione elettorale n. 2) Gallina, campo sportivo, via colombaio Vivo d'Orcia, scuola materna via 4 novembre (sezione elettorale n. 3) CETONA circolo pd, Piazza Arnolfo (sezioni elettorali n. 1 e 2)* Le Piazze, Circolo Arci Via della Resistenza (sezione elettorale n. 3) CHIANCIANO TERME Sede pd, Via Dante Alighieri* CHIUSDINO Chiusdino, Palestra ex scuola media (sez. elettorale n.1)* Palazzetto, Circolo Arci, Via Massetana (sez. elettorale n. 2) Ciciano, Circolo Arci, Via Massetana 9 ( sez. elettorale n. 4) Frassini, Circolo Arci ( sez. elettorale n. 5) Montalcinello, Circolo Unitario, Via Ugo Mancini ( sez. -

Viva Xpress Logistics (Uk)
VIVA XPRESS LOGISTICS (UK) Tel : +44 1753 210 700 World Xpress Centre, Galleymead Road Fax : +44 1753 210 709 SL3 0EN Colnbrook, Berkshire E-mail : [email protected] UNITED KINGDOM Web : www.vxlnet.co.uk Selection ZONE FULL REPORT Filter : Sort : Group : Code Zone Description ZIP CODES From To Agent IT ITAOD04 IT- 3 Days (Ex LHR) Cities & Suburbs CIVITELLA CESI 01010 - 01010 CELLERE 01010 - 01010 AZIENDA ARCIONE 01010 - 01010 ARLENA DI CASTRO 01010 - 01010 FARNESE 01010 - 01010 LATERA 01010 - 01010 MONTEROMANO 01010 - 01010 ONANO 01010 - 01010 PESCIA ROMANA 01010 - 01010 PIANSANO 01010 - 01010 TESSENNANO 01010 - 01010 VEIANO 01010 - 01010 VILLA S GIOVANNI IN TUSC 01010 - 01010 MUSIGNANO 01011 - 01011 CELLENO 01020 - 01020 CHIA 01020 - 01020 CASTEL CELLESI 01020 - 01020 CASENUOVE 01020 - 01020 GRAFFIGNANO 01020 - 01020 LUBRIANO 01020 - 01020 MUGNANO 01020 - 01020 PROCENO 01020 - 01020 ROCCALVECCE 01020 - 01020 SAN MICHELE IN TEVERINA 01020 - 01020 SERMUGNANO 01020 - 01020 SIPICCIANO 01020 - 01020 TORRE ALFINA 01020 - 01020 TREVIGNANO 01020 - 01020 TREVINANO 01020 - 01020 VETRIOLO 01020 - 01020 ACQUAPENDENTE 01021 - 01021 CIVITA BAGNOREGIO 01022 - 01022 BAGNOREGIO 01022 - 01022 CASTIGLIONE IN TEVERINA 01024 - 01024 GROTTE SANTO STEFANO 01026 - 01026 MAGUGNANO 01026 - 01026 CASTEL S ELIA 01030 - 01030 CALCATA 01030 - 01030 BASSANO ROMANO 01030 - 01030 FALERIA 01030 - 01030 FABBRICA DI ROMA 01034 - 01034 COLLEMORESCO 02010 - 02010 COLLI SUL VELINO 02010 - 02010 CITTAREALE 02010 - 02010 CASTEL S ANGELO 02010 - 02010 CASTEL SANT'ANGELO 02010 -

CHIUSDINO, MONTICIANO, SOVICILLE: Analisi Dei Contesti Territoriali
SIIC80700X - REGISTRO PROTOCOLLO - 0010762 - 05/12/2018 - C/17 accesso atti amm.vi, - E CHIUSDINO, MONTICIANO, SOVICILLE: analisi dei contesti territoriali L’Istituto A. Lorenzetti è situato al centro di un territorio variegato e ricco di storia, di cultura, di arte, di attività economiche e umane, che si è arricchito negli ultimi decenni di nuove risorse umane e etniche. I tre comuni di Chiusdino, di Monticiano e di Sovicille rappresentano tre realtà che in parte sono differenti per conformazione fisica del territorio, ma che nel tempo hanno interagito e hanno comunicato, arricchendosi a vicenda, dal punto di vista socio-culturale ed economico. Situate alle https://www.google.it/search?q=chiusdino+monticiano+boschi&espv=2&biw=1122&bih=407&source pendici della Montagnola =lnms&tbm=isch&sa=X&ved=0ahUKEwjx5_ry6LHKAhXG9w4KHdrHC4EQ_AUICCgD#imgrc=04u3aovvJ Senese, Chiusdino e PLK_M%3A Monticiano si trovano immersi in un’area boschiva di grande pregio naturalistico, mentre Sovicille è caratterizzata dalla presenza della Piana di Rosia, che porta le tracce di antichi insediamenti etruschi. Tutti e tre, con le loro diversità, sono accomunati dall’importante SIIC80700X - REGISTRO PROTOCOLLO - 0010762 - 05/12/2018 - C/17 accesso atti amm.vi, - E presenza del fiume Merse e sono collocati nella valle che dal fiume prende il nome. Il paesaggio vario – con colline, zone agricole, radure, boschi – è interessato da quattro Riserve Naturali, finalizzate alla conservazione degli ecosistemi, alla promozione e alla incentivazione delle attività produttive e del tempo libero. Tali aree sono compatibili con lo svolgimento delle attività scientifiche e di ricerca e della promozione e incentivazione delle attività coordinate di informazione e di educazione ambientale. -

Guida-Inventario Dell'archivio Di Stato
MINIS TERO PER I BENI CULTURALI E AM BIENTALI PU B BLICAZIONI DEGLI ARCHIVI DI STATO XCII ARCHIVIO DI STATO DI SIENA GUIDA-INVENTARIO DELL'ARCHIVIO DI STATO VOLUME TERZO ROMA 1977 SOMMARIO Pag. Prefazione VII Archivio Notarile l Vicariati 77 Feudi 93 Archivi privati 105 Bandini Policarpo 106 Bologna-Buonsignori-Placidi 108 Borghesi 140 Brancadori 111 Brichieri-Colombi 113 Busacca Raffaele 115 Canonica (la) 116 Nerazzini Cesare 118 Pannocchieschi-D'Elci 120 Petrucci 140 Piccolomini-Clementini 123 Piccolomini-Clementini-Adami 128 Piccolomini-Naldi-Bandini 131 Piccolomini (Consorteria) 134 Sergardi-Biringucci 137 Spannocchi 141 Tolomei 143 Useppi 146 Venturi-Gallerani 148 Particolari 151 Famiglie senesi 152 Famiglie forestiere 161 PR EFAZIONE Nella introduzione al primo volume della guida-inventario dell'Archivio di Stato di Siena furono esposti gli intenti con i quali ci si accinse alla pub blicazione di quell'importante mezzo di corredo che consistevano nel valorizzare il materiale documentario di ciascun fo ndo archivistico per facilitare agli stu diosi la via della ricerca storica. Il caloroso e benevolo accoglimento riservato dagli interessati dice chiaramente quanto fe lice sia stata l'iniziativa e quanto brillanti siano stati i risultati raggiunti, che si sono palesati anche maggiori di quelli previsti. Infa tti la valorizzazione del materiale, oltre all'ordinamento ed alla inventariazione degli atti, presupponeva anche lo studio accurato delle ori gini e delle competenze dell'ufficio o dell'ente che quegli atti aveva prodotto. Per cui, essendo stati illustrati nei primi due volumi 1 ottanta fondi appartenenti alle più importanti magistrature dello Stato, insieme alla storia di ogni singolo ufficio è emersa in breve sintesi anche la storia del popolo senese. -

Popolazione Delle Frazioni Geografiche E Delle Località Abitate Dei Comuni (20 Faseicoli 1'Egionali E Una Appenrliee Con Le Tavole Riassuntive) Voi
ISTITUTO CENTRALE DI STATISTICA 11° CENSIMENTO GENERALE DELLA POPOLAZIONE 24 OTTOBRE 1971 VOLUME III POPOLAZIONE DELLE, FRAZIONI GEOGRAFICHE E DELLE LOCALITA ABITATE, DEI COMUNI FASCICOLO 9 TOSCANA ROMA - 1974 06 74 - Contratto del 20-12-1972 - (c. 1.400) - Soc. A.B E.T.E. - Roma INDICE AVVERTENZE . Pago V PROVINCIA DI MASSA-CARRARA TAVOLA 1 - Superficie territoriale e densità - Numero delle frazioni geografiche, dei centri e dei nuclei - Popolazione residente per tipo di località abitata ......................... » 2 TAVOLA 2 - Altitudine e popolazione residente dei comuni, delle frazioni geografiche e delle località abitate . » 3 PROVINCIA DI LUCCA TAVOLA 1 - Superficie territoriale e densità - Numero delle frazioni geografiche, dei centri e dei nuclei - Popolazione residente per tipo di località abitata ......................... » 12 TAVOLA 2 - Altitudine e popolazione residente dei comuni, delle frazioni geografiche e delle località abitate ..... » 13 PROVINCIA DI PISTOIA TAVOLA 1 - Superficie territoriale e densità - Numero delle frazioni geografiche, dei centri e dei nuclei - Popolazione residente per tipo di località abitata ......................... » 30 TAVOLA 2 - Altitudine e popolazione residente dei comuni, delle frazioni geografiche e delle località abitate ... .. » 31 PROVINCIA DI FIRENZE TAVOLA 1 - Superficie territoriale e densità - Numero delle frazioni geografiche, dei centri e dei nuclei - Popolazione residente per tipo di località abitata ...... ................. » 38 TAVOLA 2 - Altitudine e popolazione residente dei comuni, delle frazioni geografiche e delle località abitate .... » 39 ì 1 t IV i lNDICE I ! I I PROVINCIA DI LIVORNO TAVOLA 1 - Superficie territoliale e jdensità - Numero delle frazioni geografiche, dei centri e ~ei nuclei - Popolazione residente per tipo di località abitata .1... !. Pago 56 I TAVOLA 2 - Altitudine e pop~~~zio~e iresidente dei comuni, delle frazioni geografiche e delle locahta ablt,te . -

Bracci Carte Sparse Testo.Pdf
MARIO BRACCI CARTE SPARSE Riflessioni, pagine di diario, relazioni, discorsi (1934-1945) Introduzione, edizione e note a cura di Stefano Moscadelli S I E N A ACCADEMIA SENESE DEGLI INTRONATI 2 0 2 0 ISBN 978-88-89073-42-1 © Tutti i diritti riservati Nessuna parte di questo libro può essere riprodotta o divulgata in qualsiasi forma e con qualsiasi mezzo elettronico, meccanico o altro senza l’autorizzazione scritta degli autori e dei proprietari dei diritti editoriali. Con il patrocinio dell’Università degli Studi di Siena e del Dipartimento di Scienze storiche e dei beni culturali INDICE Introduzione ................................................................................. 7 1. Mario Bracci: una nota biografica ................................................ 7 2. Alcuni elementi di periodizzazione ............................................... 11 3. Un’antologia di documenti ........................................................... 16 Mario Bracci, Carte sparse. Riflessioni, pagine di diario, relazioni, discorsi (1934-1945) .................................................................. 43 Collocazione dei documenti ........................................................... 45 1. Eventi e problemi di politica internazionale (12 marzo-27 luglio 1934) ......................................................... 49 2. «Cose di Spagna» [post giugno 1937] .......................................... 61 3. «Considerazioni sulla necessità e probabilità della grande guerra» (Porto S. Stefano, 3 agosto 1939) ............................................. -
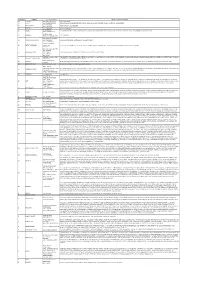
Localita' Servite Dal GPL Estra
PROVINCIA COMUNE LOCALITA'/FRAZIONE Strade in cui sono presenti pdr AR ANGHIARI LOC. PONTE ALLA PIERA PONTE ALLA PIERA AR AREZZO LOC. PALAZZO DEL PERO LOC.DUE FIUMI, LOC.PALAZZO DEL PERO, LOC.SAN DONNINO, VIA CASTIGLIONESE, VIA LOC.VALCERFONE, VIA RASSINATA LOC. FRESCIANO AR BADIA TEDALDA FRAZ. PRATIEGHI, LOC. FRESCIANO FRAZ. PRATIEGHI AR BIBBIENA LOC. TERROSSOLA VIA LOC.TERROSSOLA FRAZ. CENNINA AR BUCINE LOC. MONTEBENICHI, P.ZA DELLA CISTERNA, PIAZZA GORIZIA, VIA CAPITAN GORO, VIA DEL CASTELLO, VIA DEL FOSSO, VIA DEL POZZO, VIA IV NOVEMBRE, VICOLO DELLA FONTE FRAZ. MONTEBENICHI FRAZ. CAFAGGIO AR CAPOLONA LOC. CAFAGGIO FRAZ. PIEVE SAN GIOVANNI LOC. COLLE DI FRAGAIOLO AR CAPRESE MICHELANGELO LOC. FRAGAIOLO VIA COLLE DI FRAGAIOLO, VIA FRAGAIOLO, VIA VALBONCIONE LOC. VALBONCIONE CASTEL FOCOGNANO AR CASTEL FOCOGNANO CAPOLUOGO LOC. CASTEL FOCOGNANO, LOC. ZENNA, P.ZA DELLA LIBERTA', STRADA COMUNALE PIEVE A SOCANA, STRADA PROVINCIALE, VIA CASTELLO, VIA ROMA FRAZ. ZENNA LOC. CASE NUOVE DI DAMA LOC. COMPITO AR CHIUSI DELLA VERNA LOC. COREZZO LOC. CASE NUOVE, LOC. COMPITO, LOC. COREZZO, LOC.TA' LAPPOLA, VIA LOC.DAMA LOC. LAPPOLA LOC. DAMA CIVITELLA IN VAL DI CHIANA P.ZA BECATTINI, P.ZA MAZZINI, PIAZZA LAZZERI, VIA COSTARELLA, VIA COSTARELLINA, VIA DEI MARTIRI, VIA DEL POZZONE, VIA DELLA RIMEMBRANZA, VIA DELLE RIMEMBRANZE, VIA DI MEZZO, VIA MAESTA' TONDA, VIA NOVA, AR CIVITELLA IN VAL DI CHIANA CAPOLUOGO VIA PERTICALE, VIA SAN FRANCESCO, VIA SENESE FRAZ. MERCATALE DI AR CORTONA PIAZZA DEL MERCATO, PIAZZA DELLA COSTITUZIONE, PIAZZA SANT'ANTONIO, VIA CASALE, VIA CAVOUR, VIA DEI PONTI, VIA DEL PIGNATTAIO, VIA FILIPPO TURATI, VIA GIUSEPPE MAZZINI, VIA PIETRO DA CORTONA CORTONA AR LORO CIUFFENNA FRAZ ROCCA RICCIARDA LOC. -

Il 25 Aprile 2017 a Siena E Provincia
Il 25 aprile 2017 a Siena e provincia Il 72° anniversario della Liberazione dal Nazifascismo è ricordato a Siena e provincia con un ricco programma di manifestazioni. Ecco di seguito i principali eventi. SIENA. Lunedì 24 aprile, alle 19.30, si terrà la “66° Traversata della città”, Gara Podistica UISP SIENA da Porta Camollia a Piazza del Campo. Più fitto il programma di martedì 25. Alle 10 Trekking urbano “A spasso in città con lo storico: i monumenti di Siena tra Grande Guerra, Fascismo e Liberazione”, organizzato dall’ISRSEC partenza dalle Stanze della Me- moria, in via Malavolti, 9. Dalle 9.30 alle 18.30 Alle Stanze della Memoria, in via Malavolti, 9 Apertura straordinaria Stanze della Memoria: sarà possibile depositare la propria memoria sugli anni del fascismo e della Liberazione di Siena e provincia consegnando documenti scritti, fotografici e videointerviste. Alle 10.45 in Piazza Salimbeni si terrà un Concerto offerto dalla Banda Città del Palio diretto dal M° Giuseppe Baldesi Alle 17 CELEBRAZIONE UFFICIALE Partenza del corteo che sfilerà per le vie del centro cittadino preceduto dalla Banda Città del Palio, all’ Università di Siena, in via Banchi di Sotto 55 e alla Sinagoga, nel vicolo delle Scotte 14, verrà deposta una corona. Ore 17.45, al Teatro dei Rinnovati, Cerimonia Ufficiale Saluto del Sindaco del Comune di Siena, Bruno Valentini Saluto del Presidente della Provincia di Siena, Fabrizio Nepi Testimonianza di due alunni che hanno partecipato alla giornata regionale “Meeting della Memoria” Conclude Silvia Folchi, Presidente provinciale dell’ANPI A seguire pettacolo musicale “Bella ciao”, offerto dalla Sezione Soci di Siena di Unicoop.Fi. -

Consiglio Regionale Della Toscana ESTRATTO DAL PROCESSO VERBALE DELLA SEDUTA Del 21 Dicembre 1999
Consiglio Regionale della Toscana ESTRATTO DAL PROCESSO VERBALE DELLA SEDUTA del 21 Dicembre 1999. Presidenza del Vice Presidente del Consiglio regionale Denis Verdini. Deliberazione n. 384 concernente: L.R. 25/98 art. 9 comma 2 "Piano Regionale di gestione dei rifiuti - Terzo stralcio relativo alla bonifica delle aree inquinate". Omissis Il Presidente mette in approvazione la seguente proposta di deliberazione: IL CONSIGLIO REGIONALE omissis IL CONSIGLIO APPROVA Con la maggioranza prevista dall'art. 15 dello Statuto. IL PRESIDENTE IL SEGRETARIO Denis Verdini Enrico Bosi VISTO il D.Lgs. n. 22 del 05.02.97 e successive modifiche ed integrazioni ed in particolare l'art. 22 nel quale, al comma 7, è previsto che la Regione approva ed adegua il Piano regionale di Gestione dei rifiutifiuti entro due anni dall'entrata in vigore del decreto stesso; CONSIDERATO che, ai sensi dell'art. 22 comma 5 del citato decreto, il Piano di Bonifica delle aree inquinate costituisce parte integrante del Piano di Gestione dei rifiuti; VISTA la L.R. n. 25 del 18.05.98 “Norme per la gestione dei rifiuti e la bonifica dei siti inquinati”; VISTO l'art. 10 comma 1 della L.R. 18/05/98 n. 25 che prevede che il Piano regionale di gestione dei rifiuti è approvato dal Consiglio regionale su proposta della Giunta anche per stralci funzionali e tematici; CONSIDERATO che, ai sensi del punto precedente, gli stralci funzionali e tematici sono così individuati: primo stralcio relativo ai rifiuti urbani; secondo stralcio relativo ai rifiuti urbani e speciali; terzo stralcio relativo alla bonifica delle aree inquinate; RICORDATO che con D.C.R.T. -

REGISTRO Persone Giuridiche .Pdf
NUM. CODICE CODICE COD. FISC. DENOMINAZIONE CODICE FISCALE INDIRIZZO RAPPR. LEGALE REGISTRO COMUNE PROVINCIA RAPPR.LEGALE Ist. Interdiocesano per il sostentanento del clero di Siena-Colle di Val d'Elsa- Via di Città n. 146 Belli Fabio e BLLFNC45C09I726U 92003400527 052032 052 Montalcino e Abbazia territoriale di Siena Nencini Pietro NNCPTR64L31C847W 1 Monteoliveto Maggiore Via Pesanella n. 68 Fondazione Alimondo Ciampi Onlus 94051520487 052023 Ciampi Daniele CMPDNL55T14859L Radda in Chianti " 2 Fraz. Contignano - Mons. Icilio Parr. S. Maria Assunta 052040 " RSSCLI28S25H790W 3 Radicofani Rossi Via San Michele n. Don Sensano Parr. S. Maria della Stella 11 Chianciano 052009 " Carlo 4 Terme Giovannino Ist.Diocesano per il sostentamento del Via Fiorenzuola clero della Diocesi di Montepulciano 704650522 vecchia n. 2 052015 " Marchi Mario 5 Chiusi Montepulciano Via Berardenga n. 29 Villa Chigi - Assoc. Nazion. Città del vino 702220526 052006 Pioli Giampaolo PLIGPL48M17G687W Castelnuovo " 6 Berardenga Via Banchi di Sotto Mancini Fondaz. Monte dei Paschi di Siena 92035830526 052032 n. 34 Siena " Gabriello 7 Via di Città n. 89 Mancini Accademia musicale Chigiana 68580521 052032 PLIGPL48M17G687W 8 Siena " Gabriello Mons. Arcidiocesi di Siena , Colle di val d'Elsa Piazza Duomo n. 6 80009780521 052032 Buoncristiano BNCNTN43T20C527R e Montalcino" Siena " 9 Antonio c.f. Arcidiocesi Loc. Piana - Mons. Rosi Parr. S. Innocenzo a Piana 052003 RSOCLD55B15F676Q 10 80009780521 Buonconvento " Claudio c.f. Arcidiocesi Via Soccini n. 151 Don Poddighe Parrocchia Santi Pietro e Paoli 052003 11 80009780521 Buonconvento " Gianfranco c.f. Arcidiocesi loc. Bibbiano - Mons. Rosi Parrocchia S. Lorenzo Martire 052003 RSOCLD55B15F676Q 12 80009780521 Buonconvento " Claudio c.f. Arcidiocesi Loc Ponte d'Arbia - Mons. -

PIANO OPERATIVO Ai Sensi Della LR 65/2014
PO COMUNE DI RAPOLANO TERME Provincia di Siena PIANO OPERATIVO ai sensi della LR 65/2014 Comune di Rapolano Terme Alessandro Starnini Sindaco Elisa Morbidelli Assessore all'Urbanistica Responsabile del procedimento Sauro Malentacchi Garante dell'informazione e Partecipazione Marco Anselmi Ufficio di piano Gabriele Giardini Progettazione Urbanistica – Valutazione Ambientale Strategica Laura Tavanti Collaboratori alla progettazione Silvia Bertocci Desirè Gambini Patrizia Sodi AVVIO DEL PROCEDIMENTO DOC.A Relazione (art.17 LR 65/14, art.20-21 Disciplina di PIT/PPR) Comune di Rapolano Terme PIANO OPERATIVO AVVIO DEL PROCEDIMENTO Premessa .............................................................................................................................................................. 2 1. QUADRO DI RIFERIMENTO GENERALE ................................................................................................................ 4 1.1.Riferimenti normativi ..................................................................................................................................... 4 1.2.Il procedimento di formazione del Piano Operativo ...................................................................................... 6 1.3.Contenuti e forma del Piano Operativo (PO) ................................................................................................. 8 1.4.I contenuti del documento di avvio del procedimento ................................................................................ 10 2. DISCIPLINA URBANISTICA -

Rapporto Ambientale
VARIANTE AL REGOLAMENTO URBANISTICO “AL SERVIZIO DELLA CITTA” ___________________________________________________________________________________________________________________________________ Procedura di Valutazione Ambientale Strategica (VAS) RAPPORTO AMBIENTALE Art. 24,e Allegato 2, L.R.T. n° 10 del 12 febbraio 2010 e s.m.i. Art. 13, co.3,4,5,6 - allegatoVI, D.Lgs. n° 152 del 3 aprile 2006, e s.m.i. Gruppo di progettazione Arch. Marco Vannocci Arch. Laura Ermini Geol. Lucia Buracchini Sistema Informativo Territoriale Geom. Mauro Lusini Geom. Gabriele Comacchio Il Garante della Comunicazione Il Dirigente Dott. Gianluca Pocci (Arch. Massimo Betti) Il Responsabile del Procedimento (Arch. Rolando Valentini) Febbraio 2015 Settembre 2015 Valutazione Ambientale Strategica _ Rapporto Ambientale 1 / 191 Valutazione Ambientale Strategica FATTORI CLIMATICI, I BENI MATERIALI, IL PATRIMONIO CULTURALE, ANCHE ARCHITETTONICO E ARCHEOLOGICO, IL PAESAGGIO E L’INTERRELAZIONE TRA I SUDDETTI FATTORI ............................. 103 Rapporto Ambientale 3.6.1 Biodiversità ........................................................................................................................................................ 103 Art. 24,e Allegato 2, L.R.T. n° 10 del 12 febbraio 2010 e s.m.i. 3.6.2 Popolazione ...................................................................................................................................................... 104 3.6.3 Considerazioni sulla viabilità e sosta ............................................................................................