The Early History of Trna Recognition by Aminoacyl-Trna Synthetases
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
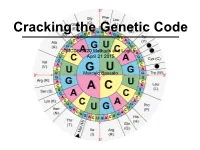
MCDB 5220 Methods and Logics April 21 2015 Marcelo Bassalo
Cracking the Genetic Code MCDB 5220 Methods and Logics April 21 2015 Marcelo Bassalo The DNA Saga… so far Important contributions for cracking the genetic code: • The “transforming principle” (1928) Frederick Griffith The DNA Saga… so far Important contributions for cracking the genetic code: • The “transforming principle” (1928) • The nature of the transforming principle: DNA (1944 - 1952) Oswald Avery Alfred Hershey Martha Chase The DNA Saga… so far Important contributions for cracking the genetic code: • The “transforming principle” (1928) • The nature of the transforming principle: DNA (1944 - 1952) • X-ray diffraction and the structure of proteins (1951) Linus Carl Pauling The DNA Saga… so far Important contributions for cracking the genetic code: • The “transforming principle” (1928) • The nature of the transforming principle: DNA (1944 - 1952) • X-ray diffraction and the structure of proteins (1951) • The structure of DNA (1953) James Watson and Francis Crick The DNA Saga… so far Important contributions for cracking the genetic code: • The “transforming principle” (1928) • The nature of the transforming principle: DNA (1944 - 1952) • X-ray diffraction and the structure of proteins (1951) • The structure of DNA (1953) How is DNA (4 nucleotides) the genetic material while proteins (20 amino acids) are the building blocks? ? DNA Protein ? The Coding Craze ? DNA Protein What was already known? • DNA resides inside the nucleus - DNA is not the carrier • Protein synthesis occur in the cytoplasm through ribosomes {• Only RNA is associated with ribosomes (no DNA) - rRNA is not the carrier { • Ribosomal RNA (rRNA) was a homogeneous population The “messenger RNA” hypothesis François Jacob Jacques Monod The Coding Craze ? DNA RNA Protein RNA Tie Club Table from Wikipedia The Coding Craze Who won the race Marshall Nirenberg J. -

Leslie E. Orgel 1927–2007
Leslie E. Orgel 1927–2007 A Biographical Memoir by Jack D. Dunitz and Gerald F. Joyce ©2013 National Academy of Sciences. Any opinions expressed in this memoir are those of the authors and do not necessarily reflect the views of the National Academy of Sciences. LESLIE ELEAZER ORGEL January 12, 1927–October 27, 2007 Elected to the NAS, 1990 Leslie Eleazer Orgel was a theoretical chemist and inves- tigator of the origins of life who made deep and lasting contributions in both of these scientific areas. He was born in London, England, on January 12, 1927, the second of three children of Simon and Deborah (Gnivisch) Orgel. His older brother Nevill was born on July 2, 1922, and died on December 28, 1957. His younger sister Delia was born on June 19, 1933, and currently resides in Silver Spring, Maryland. Leslie Orgel died on October 27, 2007, in San Diego, California, from pancreatic cancer. He is survived by his wife of 57 years, Alice (Levinson) Orgel; by his three children, Vivienne (b. April 4, 1955), Richard (b. November 29, 1956), and Robert (b. June 25, 1968); and by five By Jack D. Dunitz grandchildren. and Gerald F. Joyce After attending Dame Alice Owen’s School in London, which was evacuated during World War II to Bedford, England, Orgel studied chemistry at the University of Oxford, graduating in 1948 as BA with First Class Honours in Chem- istry. He then undertook graduate research with Leslie Sutton, senior chemistry tutor at Magdalen College and himself a distinguished physical chemist. Orgel’s1 first publication (1951) dealt with the semi-empirical calculation of electric dipole moments of conjugated heterocyclic molecules, and can be of no more than historical interest today. -

Wo 2012/064675 A2
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (10) International Publication Number (43) International Publication Date - if 18 May 2012 (18.05.2012) WO 2012/064675 A2 (51) International Patent Classification: (81) Designated States (unless otherwise indicated, for every C12N 15/11 (2006.01) C12Q 1/68 (2006.01) kind of national protection available): AE, AG, AL, AM, C07H 21/00 (2006.01) C12N 15/10 (2006.01) AO, AT, AU, AZ, BA, BB, BG, BH, BR, BW, BY, BZ, CA, CH, CL, CN, CO, CR, CU, CZ, DE, DK, DM, DO, (21) International Application Number: DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, PCT/US201 1/059656 HN, HR, HU, ID, JL, IN, IS, JP, KE, KG, KM, KN, KP, (22) International Filing Date: KR, KZ, LA, LC, LK, LR, LS, LT, LU, LY, MA, MD, 7 November 20 11 (07.1 1.201 1) ME, MG, MK, MN, MW, MX, MY, MZ, NA, NG, NI, NO, NZ, OM, PE, PG, PH, PL, PT, QA, RO, RS, RU, (25) Filing Language: English RW, SC, SD, SE, SG, SK, SL, SM, ST, SV, SY, TH, TJ, (26) Publication Language: English TM, TN, TR, TT, TZ, UA, UG, US, UZ, VC, VN, ZA, ZM, ZW. (30) Priority Data: 61/41 1,974 10 November 2010 (10.1 1.2010) US (84) Designated States (unless otherwise indicated, for every kind of regional protection available): ARIPO (BW, GH, (72) Inventor; and GM, KE, LR, LS, MW, MZ, NA, RW, SD, SL, SZ, TZ, (71) Applicant : WEBB, Nigel, L. -

Francis Crick Personal Papers
http://oac.cdlib.org/findaid/ark:/13030/kt1k40250c No online items Francis Crick Personal Papers Special Collections & Archives, UC San Diego Special Collections & Archives, UC San Diego Copyright 2007, 2016 9500 Gilman Drive La Jolla 92093-0175 [email protected] URL: http://libraries.ucsd.edu/collections/sca/index.html Francis Crick Personal Papers MSS 0660 1 Descriptive Summary Languages: English Contributing Institution: Special Collections & Archives, UC San Diego 9500 Gilman Drive La Jolla 92093-0175 Title: Francis Crick Personal Papers Creator: Crick, Francis Identifier/Call Number: MSS 0660 Physical Description: 14.6 Linear feet(32 archives boxes, 4 card file boxes, 2 oversize folders, 4 map case folders, and digital files) Physical Description: 2.04 Gigabytes Date (inclusive): 1935-2007 Abstract: Personal papers of British scientist and Nobel Prize winner Francis Harry Compton Crick, who co-discovered the helical structure of DNA with James D. Watson. The papers document Crick's family, social and personal life from 1938 until his death in 2004, and include letters from friends and professional colleagues, family members and organizations. The papers also contain photographs of Crick and his circle; notebooks and numerous appointment books (1946-2004); writings of Crick and others; film and television projects; miscellaneous certificates and awards; materials relating to his wife, Odile Crick; and collected memorabilia. Scope and Content of Collection Personal papers of Francis Crick, the British molecular biologist, biophysicist, neuroscientist, and Nobel Prize winner who co-discovered the helical structure of DNA with James D. Watson. The papers provide a glimpse of his social life and relationships with family, friends and colleagues. -

Considerations in Evolutionary Biochemistry Van Der Gulik, P.T.S
UvA-DARE (Digital Academic Repository) Considerations in evolutionary biochemistry van der Gulik, P.T.S. Link to publication Creative Commons License (see https://creativecommons.org/use-remix/cc-licenses): Other Citation for published version (APA): van der Gulik, P. T. S. (2019). Considerations in evolutionary biochemistry. Amsterdam: Institute for Logic, Language and Computation. General rights It is not permitted to download or to forward/distribute the text or part of it without the consent of the author(s) and/or copyright holder(s), other than for strictly personal, individual use, unless the work is under an open content license (like Creative Commons). Disclaimer/Complaints regulations If you believe that digital publication of certain material infringes any of your rights or (privacy) interests, please let the Library know, stating your reasons. In case of a legitimate complaint, the Library will make the material inaccessible and/or remove it from the website. Please Ask the Library: https://uba.uva.nl/en/contact, or a letter to: Library of the University of Amsterdam, Secretariat, Singel 425, 1012 WP Amsterdam, The Netherlands. You will be contacted as soon as possible. UvA-DARE is a service provided by the library of the University of Amsterdam (http://dare.uva.nl) Download date: 28 sep 2020 Considerations in Evolutionary Biochemistry Peter T. S. van der Gulik Considerations in Evolutionary Biochemistry ILLC Dissertation Series DS-2019-06 For further information about ILLC-publications, please contact Institute for Logic, Language and Computation Universiteit van Amsterdam Science Park 107 1098 XG Amsterdam phone: +31-20-525 6051 e-mail: [email protected] homepage: http://www.illc.uva.nl/ These investigations were supported by Centrum Wiskunde & Informatica (CWI), Vici grant 639-023-302 from the Netherlands Organization for Scientific Research (NWO), and the QuSoft Research Center for Quantum Software. -

Sydney Brenner's Life in Science 1927–2019
Sydney Brenner’s Life in Science 1927–2019 A Heroic Voyage: Sydney Brenner’s Life in Science Copyright © 2019 Agency for Science, Technology and Research Biomedical Research Council Agency for Science, Technology and Research 20 Biopolis Way, #08-01 Singapore 138668 All rights reserved. No part of this book may be reproduced, stored in a retrieval system or transmitted in any form or by any means, electronic, mechanical, photocopying, recording or otherwise, without the prior written permission of the Publisher. This companion booklet was originally published in conjunction with the 2015 Sydney Brenner Scientific Symposium and Exhibition, held at Singapore's Biopolis scientific hub. Agency for Science, Cold Spring Technology and Research, Harbor Laboratory, Singapore New York c A Heroic Voyage: Sydney Brenner's Life in Science ONE OF AN EXCELLENT OUR OWN TRAVEL Message by Lim Chuan Poh COMPANION Former Chairman Agency for Science, Technology and Research Message by James Watson Chancellor Emeritus Cold Spring Harbor Laboratory hirty years ago, when Sydney times to reach over S$29 billion in 2012, ver the course of my career, Then there were the exciting, frenetic Brenner first visited Singapore, contributing to 5% of Singapore’s GDP. I’ve had the privilege of years of the Human Genome Project. We the state of research, especially While Singapore’s success in this area tackling some of the most kept up our exchanges, with him in the UK in biomedical sciences, was certainly cannot be attributed to any one fundamental questions in and me in the US, doing as much science as vastly different from what it is person, few have been as deeply involved biology, working alongside possible while being responsible for entire Ttoday. -

On the Uniqueness of the Standard Genetic Code
life Article On the Uniqueness of the Standard Genetic Code Gabriel S. Zamudio and Marco V. José * Theoretical Biology Group, Instituto de Investigaciones Biomédicas, Universidad Nacional Autónoma de México, México D.F. 04510, Mexico; [email protected] * Correspondence: [email protected]; Tel.: +52-5562-3894 Academic Editor: Koji Tamura Received: 15 December 2016; Accepted: 8 February 2017; Published: 13 February 2017 Abstract: In this work, we determine the biological and mathematical properties that are sufficient and necessary to uniquely determine both the primeval RNY (purine-any base-pyrimidine) code and the standard genetic code (SGC). These properties are: the evolution of the SGC from the RNY code; the degeneracy of both codes, and the non-degeneracy of the assignments of aminoacyl-tRNA synthetases (aaRSs) to amino acids; the wobbling property; the consideration that glycine was the first amino acid; the topological and symmetrical properties of both codes. Keywords: RNY code; Standard genetic code; evolution of the genetic code; frozen code; degeneracy; aminoacyl-tRNA synthetases; symmetry 1. Introduction A fundamental feature of all life forms existing on Earth is that, with several minor exceptions, they share the same standard genetic code (SGC). This universality led Francis Crick to propose the frozen accident hypothesis [1], i.e., the SGC does not change. According to Crick [1], the SGC code remained universal because any change would be lethal, or would have been very strongly selected against and extinguished. The astonishing diversity of living beings in the history of the biosphere has not been halted by a frozen SGC. The inherent structure of the frozen SGC, in concert with environmental influences, has unleashed life from determinism. -

Diamond Code
Diamond code The Russian astrophysicist George Gamow is seen as the father of the Big Bang theory. A title he owes to his prediction of the cosmic microwave background radiation. Gamow was a creative thinker who felt quite at home taking a sidestep into another discipline. His contribution to cracking the genetic code is seen as "perhaps the last example of amateurism in scientific work on grand scale". After all, it was Gamow's idea that the base sequence in DNA might be the code for protein synthesis. In the spring of 1953, Francis Crick and James Watson decoded the structure of deoxyribonucleic acid (DNA). They discovered that DNA — the molecular basis of heredity — consists of two intertwined strands that run in opposite directions. Each strand is a long molecule comprising a string of sugars, phosphate groups, and one of the following four bases: adenine (A), thymine (T), cytosine (C) or guanine (G). The burning question was soon raised: "How is the information in DNA converted into the production of amino acids, the building blocks of proteins?". The first step in finding a solution came from an unexpected quarter. After reading the work of Watson and Crick, George Gamow wrote them a letter in the summer of 1953. He suggested that the base sequence in DNA might be the code for protein synthesis. As a physicist, Gamow's idea took the world of biology by storm. He had changed what had, until then, been seen as a chemical problem into purely a question of information storage and transfer. The underlying chemistry was of secondary importance. -

Martynas Yčas: the “Archivist” of the RNA Tie Club
| PERSPECTIVES Martynas Ycas:ˇ The “Archivist” of the RNA Tie Club Bernard S. Strauss1 Department of Molecular Genetics and Cell Biology, The University of Chicago, Illinois 60637 ORCID ID: 0000-0001-7548-6461 (B.S.S.) ABSTRACT Between about 1951 and the early 1960s, the basic structure of molecular biology was revealed. Central to our understanding was the unraveling of the various roles of RNA, culminating in the identification of messenger RNA (mRNA) and the deciphering of the genetic code. We know a great deal about the role of Brenner, Crick, Jacob, and Nirenberg in these discoveries, but many others played important supporting parts. One of these is a little-known scientist, Martynas Ycas,ˇ who appears in histories, generally without explanation, as the “archivist of the RNA Tie Club.” Ycasˇ was born in Lithuania. His father helped write the Lithuanian Constitution in 1919. He studied Roman Law and served in the Lithuanian army before escaping from the Russians in 1940. The records of correspondence of Ycasˇ with the physicist George Gamow and with Francis Crick throw some light on the genesis of our understanding of the role of mRNA. The story of the “RNA Tie Club” illustrates the difficulty in assigning credit for important discoveries and underscores the importance of a free exchange of information, even (or especially) among competitors. istory may be written by the winners, and it certainly connected in some way.The experiments of Beadle and Tatum Hemphasizes their role. Still, in 1676, or maybe 1675 de- and their followers had provided reasons for believing that a pending on whether one uses the Julian or Gregorian calendar, single gene determined, or controlled, a single enzyme (Beadle Isaac Newton wrote to Robert Hooke “If I have seen further it 1950). -

What Mad Pursuit BOOKS in the ALFRED P
What Mad Pursuit BOOKS IN THE ALFRED P. SLOAN FOUNDATION SERIES Disturbing the Universe by Freeman Dyson Advice to a Young Scientist by Peter Medawar The Youngest Science by Lewis Thomas Haphazard Reality by Hendrik B. G. Casimir In Search of Mind by Jerome Bruner A Slot Machine, a Broken Test Tube by S. E. Luria Enigmas of Chance by Mark Kac Rabi: Scientist and Citizen by John Rigden Alvarez: Adventures of a Physicist by Luis W. Alvarez Making Weapons, Talking Peace by Herbert F. York The Statue Within by François Jacob In Praise of Imperfection by Rita Levi-Montalcini Memoirs of an Unregulated Economist by George J. Stigler Astronomer by Chance by Bernard Lovell THIS BOOK IS PUBLISHED AS PART OF AN ALFRED P. SLOAN FOUNDATION PROGRAM What Mad Pursuit A Personal View of Scientific Discovery FRANCIS CRICK Library of Congress Cataloging-in-Publication Data Crick, Francis, 1916– What mad pursuit. (Alfred P. Sloan Foundation series) Includes index. 1. Crick, Francis, 1916– 2. Biologists—England—Biography. 3. Physicists—England—Biography. I. Title. II. Series. QH31.C85A3 1988 574.19’1’0924 [B] 88–47693 ISBN 0–465–09137–7 (cloth) ISBN-10: 0-465-09138-5 ISBN-13: 978-0-465-09138-6 (paper) eBook ISBN: 9780786725847 Published by BasicBooks, A Member of the Perseus Books Group Copyright © 1988 by Francis Crick Printed in the United States of America Designed by Vincent Torre Experience is the name everyone gives to their mistakes. —OSCAR WILDE Preface to the Series THE ALFRED P. SLOAN FOUNDATION has for many years had an interest in encouraging public understanding of science. -

Convergence of Physical Sciences for Biomedical Applications
Convergence of Physical Sciences for Biomedical Applications Larry A. Nagahara Associate Director Physical Sciences in Oncology Initiative Division of Cancer Biology (DCB) National Cancer Institute (NCI) National Institutes of Health (NIH) U.S. Joint Services & OSD Africa Technical Exchange Meeting, May 5-9, 2014 National Institutes of Health (NIH): 27 Institutes and Centers NHGRI NIA NIDA NINDS NIDCD NIMH NEI NIAAA CIT NINR NLM NIDDK NIH Campus – Bethesda, Maryland FIC CSR NIBIB NIMHD NIDCR NIEHS NIGMS NICHD CC NIAMS NCATS NCCAM NIAID NCI NHLBI NIH Budget ~ $30.8 Billion (FY12) NCI Budget ~ $ 5.07 Billion (FY12) ~82% for extramural support ~ 76% for extramural support ~63,000 grants and contracts ~7,800 grants and contracts National Cancer Institute Organization National Cancer Institute $5.07B Director (FY12) Office of the Deputy Director Harold Varmus, MD Director Douglas Lowy, MD Nobel Prize (1989) CSSI OPSO Center for Strategic (2013) ~$132 M (~4%) Scientific Initiatives Division of Division of Division of Center for Cancer Division of Cancer Division of Division of Cancer Cancer Treatment Cancer Control and Cancer Extramural Epidemiology Research and Population Prevention Activities and Genetics Biology Diagnosis Sciences ~$858M (~17%) ~$919M (~29%) ~$779M (~25%) ~$441M (~14%) ~$264M (~8%) ~$21M (~0.4%) Conducting – Intramural Funding – Extramural Red numbers: FY12 grants data only from http://fundedresearch.cancer.gov/nciportfolio; Black numbers: from FY12 http://obf.cancer.gov/financial/factbook.htm Cancer Global Effect – Incidence/Mortality 7.6 million people died of cancer in 2005 (out of 58 millions deaths) >70% in low and medium income countries New cancer cases in the US will approach 2 million by 2025 Source: International Agency for Research on Cancer: GLOBOCAN 2008 Database Cancer Statistics between South Africa and US South Africa US What is It? Tumor, Cancer, and Metastasis “…>90% of deaths is caused by disseminated disease or metastasis…” Gupta et. -

Uno De Los Nuestros Descifrando El Código
UNO DE LOS NUESTROS DESCIFRANDO EL CÓDIGO GENÉTICO O cómo pasar de un lenguaje de 4 letras a uno de 20 Pedro García Área de Genética, Departamento de Biología Molecular, Facultad de CC. Biológicas y Ambientales, Universidad de León. Aunque siempre es buen momento para recordar cómo se han realizado los descubrimientos que han marcado la Biología actual, la celebración de un aniversario "redondo" nos proporciona una excusa perfecta. En este 2018 se cumplen 50 años desde que se concedió el premio Nobel en Fisiología o Medicina a tres investigadores por su interpretación del código genético y su función en la síntesis de proteínas: Marshall Niremberg, Har Gobind Khorana y Robert W. Holley. En esta historia también tuvieron un papel decisivo otros muchos cien- tíficos, varios de los cuales habían obtenido el premio Nobel previamente como Severo Ochoa, James D. Watson y Francis H. C. Crick, o lo obtendrían poste- riormente como Sydney Brenner. A lo largo del presente trabajo repasaremos brevemente cómo se consi- guió establecer la verdadera relación que existe entre los genes y las proteínas, la aportación del numeroso grupo de investigadores implicados al desciframiento del código, en especial la de los tres premiados en 1968, y por último algunos nue- vos conocimientos que se han obtenido sobre este tema desde dicho año. La relación entre genes y proteínas antes de la doble hélice Desde el nacimiento de la Genética como ciencia en 1900, con el redescu- brimiento de los trabajos de Mendel por Carl Correns, Hugo de Vries y Erich von Tschermak, se aceptó que las características que presentan los organismos ve- nían determinadas por unas unidades misteriosas que se denominaron genes.