Environmental Perturbations Lift the Degeneracy of the Genetic Code To
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Recombination and the Evolution of Mutational Robustness
ARTICLE IN PRESS Journal of Theoretical Biology 241 (2006) 707–715 www.elsevier.com/locate/yjtbi Recombination and the evolution of mutational robustness Andy Gardnera,b,c,Ã, Alex T. Kalinkaa aInstitute of Evolutionary Biology, University of Edinburgh, Edinburgh EH9 3JT, UK bDepartment of Mathematics & Statistics, Queen’s University, Kingston, Ont., Canada K7L 3N6 cDepartment of Biology, Queen’s University, Kingston, Ont., Canada K7L 3N6 Received 25 July 2005; received in revised form 8 December 2005; accepted 5 January 2006 Available online 20 February 2006 Abstract Mutational robustness is the degree to which a phenotype, such as fitness, is resistant to mutational perturbations. Since most of these perturbations will tend to reduce fitness, robustness provides an immediate benefit for the mutated individual. However, robust systems decay due to the accumulation of deleterious mutations that would otherwise have been cleared by selection. This decay has received very little theoretical attention. At equilibrium, a population or asexual lineage is expected to have a mutation load that is invariant with respect to the selection coefficient of deleterious alleles, so the benefit of robustness (at the level of the population or asexual lineage) is temporary. However, previous work has shown that robustness can be favoured when robustness loci segregate independently of the mutating loci they act upon. We examine a simple two-locus model that allows for intermediate rates of recombination and inbreeding to show that increasing the effective recombination rate allows for the evolution of greater mutational robustness. r 2006 Elsevier Ltd. All rights reserved. Keywords: Canalization; Epistasis; Linkage disequilibrium; Multilocus methodology; Mutation–selection balance 1. -

Antigen-Receptor Degeneracy and Immunological Paradigms Irun R
Molecular Immunology 40 (2004) 993–996 Antigen-receptor degeneracy and immunological paradigms Irun R. Cohen a,∗, Uri Hershberg b, Sorin Solomon c a The Department of Immunology, The Weizmann Institute of Science, Rehovot, 76100 Israel b Interdisciplinary Center for Neuronal Computation, The Hebrew University of Jerusalem, Jerusalem, Israel c The Racah Institute of Physics, The Hebrew University of Jerusalem, Jerusalem, Israel Abstract This paper discusses some consequences of the discovery that antigen receptors are degenerate: Immune specificity, in contrast to the tenets of the clonal selection paradigm, must be generated by the immune response down-stream of initial antigen recognition; and specificity is a property of a collective of cells and not of single clones. © 2003 Elsevier Ltd. All rights reserved. Keywords: T cells; T-cell receptor; Inflammation; Specificity; Degeneracy; Clonal selection; Cognitive paradigm 1. Degeneracy problems characterized by extreme poly-clonality; in fact, most nat- ural T-cell responses are oligo-clonal (Douek et al., 2003). Degeneracy, in the present discourse, refers to the capac- So, there must be a mechanism or mechanisms that operate ity of any single antigen receptor to bind and respond to to restrict poly-clonality in wild-type adaptive immune sys- (recognize) many different ligands. The Oxford English Dic- tems. The inherent potential for extreme poly-clonality, in tionary (Second Edition, 1989) defines the primary meaning practice, may be tempered by clonal competition. Compe- of degeneracy as: tition among clones for access, energy or space will most likely reward the fast and the avid. Since the best clones Having lost the qualities proper to the race or kind; having should win, clonal competition does not threaten the logic declined from a higher to a lower type; hence, declined of the clonal selection theory (CST) of adaptive immunity. -

Multiple Receptor Tyrosine Kinases Are Expressed in Adult Rat Retinal Ganglion Cells As Revealed by Single-Cell Degenerate Primer Polymerase Chain Reaction
Upsala Journal of Medical Sciences. 2010; 115: 65–80 ORIGINAL ARTICLE Multiple receptor tyrosine kinases are expressed in adult rat retinal ganglion cells as revealed by single-cell degenerate primer polymerase chain reaction NICLAS LINDQVIST1, ULRIKA LÖNNGREN1, MARTA AGUDO2,3, ULLA NÄPÄNKANGAS1, MANUEL VIDAL-SANZ2 & FINN HALLBÖÖK1 1Department of Neuroscience, Unit for Developmental Neuroscience, Biomedical Center, Uppsala University, 75123 Uppsala, Sweden, 2Departamento de Oftalmología, Facultad de Medicina, Universidad de Murcia, Murcia, Spain, and 3Fundación para la Formación e Investigación Sanitaria de la Región de Murcia, Hospital Universitario Virgen de la Arrixaca, Murcia, Spain Abstract Background. To achieve a better understanding of the repertoire of receptor tyrosine kinases (RTKs) in adult retinal ganglion cells (RGCs) we performed polymerase chain reaction (PCR), using degenerate primers directed towards conserved sequences in the tyrosine kinase domain, on cDNA from isolated single RGCs univocally identified by retrograde tracing from the superior colliculi. Results. All the PCR-amplified fragments of the expected sizes were sequenced, and 25% of them contained a tyrosine kinase domain. These were: Axl, Csf-1R, Eph A4, Pdgfrb, Ptk7, Ret, Ros, Sky, TrkB, TrkC, Vegfr-2, and Vegfr-3. Non-RTK sequences were Jak1 and 2. Retinal expression of Axl, Csf-1R, Pdgfrb, Ret, Sky, TrkB, TrkC, Vegfr-2, and Vegfr-3, as well as Jak1 and 2, was confirmed by PCR on total retina cDNA. Immunodetection of Csf-1R, Pdgfra/b, Ret, Sky, TrkB, and Vegfr-2 on retrogradely traced retinas demonstrated that they were expressed by RGCs. Co-localization of Vegfr-2 and Csf-1R, of Vegfr-2 and TrkB, and of Csf-1R and Ret in retrogradely labelled RGCs was shown. -

Degeneracy in Hippocampal Physiology and Plasticity
bioRxiv preprint doi: https://doi.org/10.1101/203943; this version posted July 30, 2018. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. Degeneracy in hippocampal physiology and plasticity * Rahul Kumar Rathour and Rishikesh Narayanan Cellular Neurophysiology Laboratory, Molecular Biophysics Unit, Indian Institute of Science, Bangalore, India. * Corresponding Author Rishikesh Narayanan, Ph.D. Molecular Biophysics Unit Indian Institute of Science Bangalore 560 012, India. e-mail: [email protected] Phone: +91-80-22933372 Fax: +91-80-23600535 Abbreviated title: Degeneracy in the hippocampus Keywords: hippocampus; degeneracy; learning; memory; encoding; homeostasis; plasticity; physiology; causality; reductionism; holism; structure-function relationships; variability; compensation; intrinsic excitability 1 bioRxiv preprint doi: https://doi.org/10.1101/203943; this version posted July 30, 2018. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. ABSTRACT Degeneracy, defined as the ability of structurally disparate elements to perform analogous function, has largely been assessed from the perspective of maintaining robustness of physiology or plasticity. How does the framework of degeneracy assimilate into an encoding system where the ability to change is an essential ingredient for storing new incoming information? Could degeneracy maintain the balance between the apparently contradictory goals of the need to change for encoding and the need to resist change towards maintaining homeostasis? In this review, we explore these fundamental questions with the mammalian hippocampus as an example encoding system. We systematically catalog lines of evidence, spanning multiple scales of analysis, that demonstrate the expression of degeneracy in hippocampal physiology and plasticity. -

An Interdisciplinary Perspective on Artificial Immune Systems
Evol. Intel. (2008) 1:5–26 DOI 10.1007/s12065-007-0004-2 REVIEW ARTICLE An interdisciplinary perspective on artificial immune systems J. Timmis Æ P. Andrews Æ N. Owens Æ E. Clark Received: 7 September 2007 / Accepted: 11 October 2007 / Published online: 10 January 2008 Ó Springer-Verlag 2008 Abstract This review paper attempts to position the area 1 Introduction of Artificial Immune Systems (AIS) in a broader context of interdisciplinary research. We review AIS based on an Artificial Immune Systems (AIS) is a diverse area of established conceptual framework that encapsulates math- research that attempts to bridge the divide between ematical and computational modelling of immunology, immunology and engineering and are developed through abstraction and then development of engineered systems. the application of techniques such as mathematical and We argue that AIS are much more than engineered systems computational modelling of immunology, abstraction from inspired by the immune system and that there is a great those models into algorithm (and system) design and deal for both immunology and engineering to learn from implementation in the context of engineering. Over recent each other through working in an interdisciplinary manner. years there have been a number of review papers written on AIS with the first being [25] followed by a series of others Keywords Artificial immune systems Á that either review AIS in general, for example, [29, 30, 43, Immunological modelling Á Mathematical modelling Á 68, 103], or more specific aspects of AIS such as data Computational modelling Á mining [107], network security [71], applications of AIS Applications of artificial immune systems Á [58], theoretical aspects [103] and modelling in AIS [39]. -

Degeneracy and Genetic Assimilation in RNA Evolution Reza Rezazadegan1* and Christian Reidys1,2
Rezazadegan and Reidys BMC Bioinformatics (2018) 19:543 https://doi.org/10.1186/s12859-018-2497-3 RESEARCH ARTICLE Open Access Degeneracy and genetic assimilation in RNA evolution Reza Rezazadegan1* and Christian Reidys1,2 Abstract Background: The neutral theory of Motoo Kimura stipulates that evolution is mostly driven by neutral mutations. However adaptive pressure eventually leads to changes in phenotype that involve non-neutral mutations. The relation between neutrality and adaptation has been studied in the context of RNA before and here we further study transitional mutations in the context of degenerate (plastic) RNA sequences and genetic assimilation. We propose quasineutral mutations, i.e. mutations which preserve an element of the phenotype set, as minimal mutations and study their properties. We also propose a general probabilistic interpretation of genetic assimilation and specialize it to the Boltzmann ensemble of RNA sequences. Results: We show that degenerate sequences i.e. sequences with more than one structure at the MFE level have the highest evolvability among all sequences and are central to evolutionary innovation. Degenerate sequences also tend to cluster together in the sequence space. The selective pressure in an evolutionary simulation causes the population to move towards regions with more degenerate sequences, i.e. regions at the intersection of different neutral networks, and this causes the number of such sequences to increase well beyond the average percentage of degenerate sequences in the sequence space. We also observe that evolution by quasineutral mutations tends to conserve the number of base pairs in structures and thereby maintains structural integrity even in the presence of pressure to the contrary. -

Tyrosine Kinases
KEVANM SHOKAT MINIREVIEW Tyrosine kinases: modular signaling enzymes with tunable specificities Cytoplasmic tyrosine kinases are composed of modular domains; one (SHl) has catalytic activity, the other two (SH2 and SH3) do not. Kinase specificity is largely determined by the binding preferences of the SH2 domain. Attaching the SHl domain to a new SH2 domain, via protein-protein association or mutation, can thus dramatically change kinase function. Chemistry & Biology August 1995, 2:509-514 Protein kinases are one of the largest protein families identified, This is a result of the overlapping substrate identified to date; over 45 new members are identified specificities of many tyrosine kinases, which makes it each year. It is estimated that up to 4 % of vertebrate pro- difficult to dissect the individual signaling pathways by teins are protein kinases [l].The protein kinases are cate- scanning for unique target motifs [2]. gorized by their specificity for serineithreonine, tyrosine, or histidine residues. Protein tyrosine kinases account for The apparent promiscuity of individual tyrosine kinases roughly half of all kinases. They occur as membrane- is a result of their unique structural organization. bound receptors or cytoplasmic proteins and are involved Enzyme specificity is typically programmed by one in a wide variety of cellular functions, including cytokine binding site, which recognizes the substrate and also con- responses, antigen-dependent immune responses, cellular tains exquisitely oriented active-site functional groups transformation by RNA viruses, oncogenesis, regulation that help to lower the energy of the transition state for of the cell cycle, and modification of cell morphology the conversion of specific substrates to products.Tyrosine (Fig. -
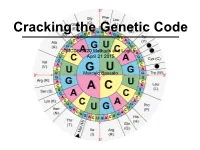
MCDB 5220 Methods and Logics April 21 2015 Marcelo Bassalo
Cracking the Genetic Code MCDB 5220 Methods and Logics April 21 2015 Marcelo Bassalo The DNA Saga… so far Important contributions for cracking the genetic code: • The “transforming principle” (1928) Frederick Griffith The DNA Saga… so far Important contributions for cracking the genetic code: • The “transforming principle” (1928) • The nature of the transforming principle: DNA (1944 - 1952) Oswald Avery Alfred Hershey Martha Chase The DNA Saga… so far Important contributions for cracking the genetic code: • The “transforming principle” (1928) • The nature of the transforming principle: DNA (1944 - 1952) • X-ray diffraction and the structure of proteins (1951) Linus Carl Pauling The DNA Saga… so far Important contributions for cracking the genetic code: • The “transforming principle” (1928) • The nature of the transforming principle: DNA (1944 - 1952) • X-ray diffraction and the structure of proteins (1951) • The structure of DNA (1953) James Watson and Francis Crick The DNA Saga… so far Important contributions for cracking the genetic code: • The “transforming principle” (1928) • The nature of the transforming principle: DNA (1944 - 1952) • X-ray diffraction and the structure of proteins (1951) • The structure of DNA (1953) How is DNA (4 nucleotides) the genetic material while proteins (20 amino acids) are the building blocks? ? DNA Protein ? The Coding Craze ? DNA Protein What was already known? • DNA resides inside the nucleus - DNA is not the carrier • Protein synthesis occur in the cytoplasm through ribosomes {• Only RNA is associated with ribosomes (no DNA) - rRNA is not the carrier { • Ribosomal RNA (rRNA) was a homogeneous population The “messenger RNA” hypothesis François Jacob Jacques Monod The Coding Craze ? DNA RNA Protein RNA Tie Club Table from Wikipedia The Coding Craze Who won the race Marshall Nirenberg J. -

Leslie E. Orgel 1927–2007
Leslie E. Orgel 1927–2007 A Biographical Memoir by Jack D. Dunitz and Gerald F. Joyce ©2013 National Academy of Sciences. Any opinions expressed in this memoir are those of the authors and do not necessarily reflect the views of the National Academy of Sciences. LESLIE ELEAZER ORGEL January 12, 1927–October 27, 2007 Elected to the NAS, 1990 Leslie Eleazer Orgel was a theoretical chemist and inves- tigator of the origins of life who made deep and lasting contributions in both of these scientific areas. He was born in London, England, on January 12, 1927, the second of three children of Simon and Deborah (Gnivisch) Orgel. His older brother Nevill was born on July 2, 1922, and died on December 28, 1957. His younger sister Delia was born on June 19, 1933, and currently resides in Silver Spring, Maryland. Leslie Orgel died on October 27, 2007, in San Diego, California, from pancreatic cancer. He is survived by his wife of 57 years, Alice (Levinson) Orgel; by his three children, Vivienne (b. April 4, 1955), Richard (b. November 29, 1956), and Robert (b. June 25, 1968); and by five By Jack D. Dunitz grandchildren. and Gerald F. Joyce After attending Dame Alice Owen’s School in London, which was evacuated during World War II to Bedford, England, Orgel studied chemistry at the University of Oxford, graduating in 1948 as BA with First Class Honours in Chem- istry. He then undertook graduate research with Leslie Sutton, senior chemistry tutor at Magdalen College and himself a distinguished physical chemist. Orgel’s1 first publication (1951) dealt with the semi-empirical calculation of electric dipole moments of conjugated heterocyclic molecules, and can be of no more than historical interest today. -

Wo 2012/064675 A2
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (10) International Publication Number (43) International Publication Date - if 18 May 2012 (18.05.2012) WO 2012/064675 A2 (51) International Patent Classification: (81) Designated States (unless otherwise indicated, for every C12N 15/11 (2006.01) C12Q 1/68 (2006.01) kind of national protection available): AE, AG, AL, AM, C07H 21/00 (2006.01) C12N 15/10 (2006.01) AO, AT, AU, AZ, BA, BB, BG, BH, BR, BW, BY, BZ, CA, CH, CL, CN, CO, CR, CU, CZ, DE, DK, DM, DO, (21) International Application Number: DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, PCT/US201 1/059656 HN, HR, HU, ID, JL, IN, IS, JP, KE, KG, KM, KN, KP, (22) International Filing Date: KR, KZ, LA, LC, LK, LR, LS, LT, LU, LY, MA, MD, 7 November 20 11 (07.1 1.201 1) ME, MG, MK, MN, MW, MX, MY, MZ, NA, NG, NI, NO, NZ, OM, PE, PG, PH, PL, PT, QA, RO, RS, RU, (25) Filing Language: English RW, SC, SD, SE, SG, SK, SL, SM, ST, SV, SY, TH, TJ, (26) Publication Language: English TM, TN, TR, TT, TZ, UA, UG, US, UZ, VC, VN, ZA, ZM, ZW. (30) Priority Data: 61/41 1,974 10 November 2010 (10.1 1.2010) US (84) Designated States (unless otherwise indicated, for every kind of regional protection available): ARIPO (BW, GH, (72) Inventor; and GM, KE, LR, LS, MW, MZ, NA, RW, SD, SL, SZ, TZ, (71) Applicant : WEBB, Nigel, L. -

Francis Crick Personal Papers
http://oac.cdlib.org/findaid/ark:/13030/kt1k40250c No online items Francis Crick Personal Papers Special Collections & Archives, UC San Diego Special Collections & Archives, UC San Diego Copyright 2007, 2016 9500 Gilman Drive La Jolla 92093-0175 [email protected] URL: http://libraries.ucsd.edu/collections/sca/index.html Francis Crick Personal Papers MSS 0660 1 Descriptive Summary Languages: English Contributing Institution: Special Collections & Archives, UC San Diego 9500 Gilman Drive La Jolla 92093-0175 Title: Francis Crick Personal Papers Creator: Crick, Francis Identifier/Call Number: MSS 0660 Physical Description: 14.6 Linear feet(32 archives boxes, 4 card file boxes, 2 oversize folders, 4 map case folders, and digital files) Physical Description: 2.04 Gigabytes Date (inclusive): 1935-2007 Abstract: Personal papers of British scientist and Nobel Prize winner Francis Harry Compton Crick, who co-discovered the helical structure of DNA with James D. Watson. The papers document Crick's family, social and personal life from 1938 until his death in 2004, and include letters from friends and professional colleagues, family members and organizations. The papers also contain photographs of Crick and his circle; notebooks and numerous appointment books (1946-2004); writings of Crick and others; film and television projects; miscellaneous certificates and awards; materials relating to his wife, Odile Crick; and collected memorabilia. Scope and Content of Collection Personal papers of Francis Crick, the British molecular biologist, biophysicist, neuroscientist, and Nobel Prize winner who co-discovered the helical structure of DNA with James D. Watson. The papers provide a glimpse of his social life and relationships with family, friends and colleagues. -

A Numerical Representation and Classification of Codons To
bioRxiv preprint doi: https://doi.org/10.1101/2020.03.02.971036; this version posted March 3, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. A Numerical Representation and Classication of Codons to Investigate Codon Alternation Patterns during Genetic Mutations on Disease Pathogenesis Antara Senguptaa, Pabitra Pal Choudhuryd, Subhadip Chakrabortyb,e, Swarup Royc,∗∗, Jayanta Kumar Dasd,∗, Ditipriya Mallicke, Siddhartha S Janae aDepartment of Master of Computer Applications, MCKV Institute of Engineering, Liluah, India bDepartment of Botany, Nabadwip Vidyasagar College, Nabadwip, India cDepartment of Computer Applications,Sikkim University, Gangtok, Sikkim, India dApplied Statistical Unit, Indian Statistical Institute, Kolkata, India eSchool of Biological Sciences, Indian Association for the Cultivation of Science, Kolkata, India 1. Introduction Genes are the functional units of heredity [4]. It is mainly responsible for the structural and functional changes and for the variation in organisms which could be good or bad. DNA (Deoxyribose Nucleic Acid) sequences build the genes of organisms which in turn encode for particular protein us- ing codon. Any uctuation in this sequence (codons), for example, mishaps during DNA transcription, might lead to a change in the genetic code which alter the protein synthesis. This change is called mutation. Mutation diers from Single Nucleotide Polymorphism(SNP) in many ways [23]. For instance, occurrence of mutation in a population should be less than 1% whereas SNP occurs with greater than 1%. Mutation always occurs in diseased group whereas SNP is occurs in both diseased and control population. Mutation is responsible for some disease phenotype but SNP may or may not be as- sociated with disease phenotype.