Reuse of Aqueous Waste Streams in Transportation Related Applications
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Buttermaking on the Farm
O F E S O D AIR" CHIEF F IC R F THE AND COLD STORAGE ' mm s n . A UDDICK s J . R Co i io er . , ' ’ v r r . J N L N hi f o f i rke t n d . I EI O C e D s D a "a s a l Sto . S G C . , i i ion y o d age F - v o f a P . Chief , Di isi n o D iry roduce C v s n r R esea rc h hief , Di i io of Dai y En fo rc em en t In Charge , of Dairy La ws . I C . n harge , Milk Utilization Service S n r a r P u e ra d H H C G . OS J . I e io D i y rod c er T . PRINC IP AL SERVICES ASSIG NED TO THE STORAG E BR ANCH ( 1 ) Grading of D a iry Prod uc e ; ( 2 ) Scien tific R esea r ch i n ’ Study of World s Co n ditions in Dairying ; (4) C orr espondence a l l M atte rs relating to D a iry ing ; ( 5 ) In specti on of Perish a b Can adia n a n d United Kin gdom Po rts ; (6 ) R efrigera to r Ca r D airy Ma rket Intelligence ; (8) Pr omoting Uniformity in 5- n B d ee t "h b on ( 9 ) Ji1 d gi g utter an Ch se a E i iti s ; (10) Cold Storag e Ac t a nd Creamery C ol d Stora ge B onuses ; ” D a i L s a n d ( 1 2 the on an d Its Pr ry aw , ) Utilizati of Milk a Dairy butter as defined by The D iry Industry Act , is butter t a m ade from the milk of less h n fifty cows . -

ABC Butter Making, by Burch 30 Harris' Cheese and Butter Maker's Hand Book 1 50 the Jersey, Alderney Aud Ouernsey Cow 1 75 Feeding Animals
ABC BUTTER MAKING Hand-Book for the Beginner. BY F. S. BUI^CH, Editok of The Dairy World. CHICAGO : C. S. BuRCH Publishing Company. 1888. 6S9 Entered according- lo Act of Congress, in the year 1888, by F. S. BURCH, In the Office of the Librarian of Congress, at ^Vashington, D. C. CONTENTS. Page MlIiKING 17 Washing the Udder—The Slow Milker — The Jerky Milker—Best Time to Milk- Kicking Cows—Feeding during the Milking — Loud Talking — Milking Tubes — The Stool—The Pail. Cake of Milk 23 Animal Heat — Milk as an Absorbant — Stable Odors—Cooling—Keeping in Pantry or Cellar—Deep Setting—Temperature of the Water—To Raise Cream Quickly—When to Skim. The Milk Room 27 To have well Ventilated—Controlling the Temperature—Pure Air —Management of Cream—Stirring the Cream—Proper Tem- perature at which to keep Cream—Ripen- ing Cream—Straining Cream—Cream in Winter. Butter Color • • • 30 Rich Orange Color — White butter —The — X CONTENTS. Page Juice of Carrots—The Use of Annato—Com- mercial Colors—Beginners generally use too much. Churning 32 The Patent Lightning Churn—Churning too Quickly—The amount of time to prop- erly do the Work—Churning Cream at 60 degrees—Winter Churning — Starting the Churn at a Slow Movement—The Churn with a Dasher—Stopping at the proper time —Granular Butter—Draining off the Butter- milk—Washing in the Churn—To have the . Churn sufficiently Large—Churning whole Milk—The Best Churn for the Dairy. WOEKING THE BuTTEE 38 The Right Temperature—To get the Butter- milk all out—Half Worked Butter—Over- working—Use of the Lever—Working in the Salt—Rule for Salting—Butter Salting Scales. -
![IS 253 (1985): Edible Common Salt [FAD 8: Food Additives]](https://docslib.b-cdn.net/cover/4909/is-253-1985-edible-common-salt-fad-8-food-additives-1274909.webp)
IS 253 (1985): Edible Common Salt [FAD 8: Food Additives]
इंटरनेट मानक Disclosure to Promote the Right To Information Whereas the Parliament of India has set out to provide a practical regime of right to information for citizens to secure access to information under the control of public authorities, in order to promote transparency and accountability in the working of every public authority, and whereas the attached publication of the Bureau of Indian Standards is of particular interest to the public, particularly disadvantaged communities and those engaged in the pursuit of education and knowledge, the attached public safety standard is made available to promote the timely dissemination of this information in an accurate manner to the public. “जान का अधकार, जी का अधकार” “परा को छोड न 5 तरफ” Mazdoor Kisan Shakti Sangathan Jawaharlal Nehru “The Right to Information, The Right to Live” “Step Out From the Old to the New” IS 253 (1985): edible common salt [FAD 8: Food Additives] “ान $ एक न भारत का नमण” Satyanarayan Gangaram Pitroda “Invent a New India Using Knowledge” “ान एक ऐसा खजाना > जो कभी चराया नह जा सकताह ै”ै Bhartṛhari—Nītiśatakam “Knowledge is such a treasure which cannot be stolen” xxxx 2009 IS : 253 - 1985 Indian Standard SPECIFICATION FOR EDIBLE COMMON SALT ( Third Revision) Acids, Alkalis and Halides Sectional Committee, CDC 56 Chairman Refiresen t ing PROF M. M. TAQUI KHA~V Central Salt and Marine Chemicals Research Institute ( CSIR ), Bhavnagar Members DR G. D. BHAT ( Alt#matc to Prof M. M. Taqui Khan ) SHRI B. K. ANAND Punjab National Fertilizers and Chemicals Ltd, Chandigarh SHRI R. -

Minnesota Rules 1989
MINNESOTA RULES 1989 1147 FOOD DEFINITIONS AND STANDARDS CHAPTER 1555 DEPARTMENT OF AGRICULTURE FOOD DEFINITIONS AND STANDARDS ORGANIC FOOD 1555.0320 OPTIONAL BLEACHING 1555.0005 DEFINITIONS. INGREDIENTS. 1555.0006 ORGANIC FOOD REQUIREMENTS. 1555.0330 LABEL STATEMENT OF 1555.0007 EXCEPTIONS. OPTIONAL INGREDIENTS. 1555.0008 TREATED SEED. TRANSPLANTS 1555.0340 DETERMINATION OF ASH. AND PROPAGATING PARTS. PROTEIN, AND MOISTURE. 1555.0009 RECORDS. 1555.0350 ENRICHED FLOUR. 1555.0010 INSPECTION AUTHORITY. 1555.0360 BROMATED FLOUR. 1555.0011 CERTIFICATION OR 1555.0370 ENRICHED BROMATED FLOUR. VERIFICATION. 1555.0380 DURUM FLOUR. 1555.0012 PROHIBITIONS. 1555.0390 SELF-RISING FLOUR. SELF- 1555.0013 EFFECTIVE DATES. RISING WHITE FLOUR, SELF- CACAO PRODUCTS RISING WHEAT FLOUR. 1555.0020 CACAO NIBS, COCOA NIBS, I 555.0400 OPTIONAL BLEACHING CRACKED COCOA. INGREDIENTS LABELING. 1555.0030 OPTIONAL ALKALI 1555.0410 METHOD. INGREDIENTS. 1555.0420 ENRICHED SELF-RISING FLOUR. 1555.0040 CHOCOLATE LIQUOR. 1555.0430 PHOSPHATED FLOUR, CHOCOLATE, BAKING PHOSPHATED WHITE FLOUR. CHOCOLATE, BITTER CHOCOLATE, COOKING PHOSPHATED WHEAT FLOUR. 1555.0440 CHOCOLATE, CHOCOLATE WHOLE WHEAT FLOUR. COATING, BITTER CHOCOLATE GRAHAM FLOUR, ENTIRE COATING. WHEAT FLOUR. 1555.0050 OPTIONAL INGREDIENTS. 1555.0450 LABEL STATEMENT OF 1555.0060 LABEL STATEMENT OF OPTIONAL BLEACHING OPTIONAL INGREDIENTS. INGREDIENT. 1555.0070 OPTIONAL ALKALI INGREDIENT. 1555.0460 DETERMINATION OF MOISTURE. 1555.0080 BREAKFAST COCOA. HIGH FAT 1555.0470 METHOD. COCOA. 1555.0480 BROMATED WHOLE WHEAT 1555.0090 OPTIONAL INGREDIENTS. FLOUR. 1555.0100 LABEL STATEMENT OF 1555.0490 WHOLE DURUM WHEAT FLOUR. OPTIONAL INGREDIENTS. 1555.0500 CRUSHED WHEAT, COARSE 1555.0110 OPTIONAL ALKALI INGREDIENT. GROUND WHEAT. 1555.0120 COCOA, MEDIUM FAT COCOA. 1555.0510 CRACKED WHEAT. 1555.0130 LOW-FAT COCOA. -

1953 Chapter 97
1707 DAIRY, FOODS AND DRUGS 97.02 CHAPTER 97. DAIRY, FOODS AND DRUGS. 97.01 Foods and drugs; definitions. 97.40 Insanitary by-products; sale, prohib 97.02 Food products; definitions; standards. ited. 97.022 Regulations. 97.41 Butter and cheese manufacturers; ac- 97.023 Lobbying on food regulations. counts accessible. 97.025 Imitation ice cream or ices. 97.42 Oleomargarine regulations. 97.03 Buttermaker and cheesemaker li- 97.43 Butter; grading; label. cense. 97.44 Notice of sale of imitation butter. 97.035 Limburger cheese; improving quality. 97.45 Renovated butter. 97.04 Dairy plants and receiving stations. 97.46 Frauds; substitute for butter; adver 97.045 Milk pasteurization; licensing of milk ti 8ement. dealers. 97.47 Imitation dairy products in public in- 97.05 Coun tel' freezers licensed. stitutions. 97.06 Food processor's license. 97.48 High moisture and low fat cheese. 97.08 Special dairy and food inspectors. 97.49 Excess moisture cheese. 97.09 Regulation of soda water business. 97.50 Cheese food compounds. 97.10 Bakery license. 97.51 Cheese boxes must be clean. 97.11 Confectionary license. 97.52 Fraud in labeling cheese. 97.12 Bakery and confectionary license re 97.53 Adulteration of meats. quirements. 97.54 Sausage; sausage mixture. 97.25 Drugs and foods; adulteration. 97.55 Meat from dead or diseased animals. 9J .26 Foods; manufacture; sale; sanitary 97.555 Labeling of horsemeat. regulations. 97.56 Kosher meat. 97.27 Sale of certain foods regulated and 97.57 Buckwheat flour compound. restricted. 97.59 Impure ice. 97.275 Sale of rosefish. -

1. Introduction: Salt, Sodium Chloride, Has Probably Been with Us from The
1. Introduction: Salt, sodium chloride, has probably been with us from the beginnings of geologic time, and has been necessary directly or indirectly through all stages of evolution of living things. The first oceans undoubtedly contained traces of salt, and our unicellular ancestors first appeared and thrived in this salty marine environment. In the process of evolution, these unicellular ancestors became multi-cellular, and some left their salty marine environment but still required salt. Our herbivorous ancestors used salt licks, and our carnivorous ones obtained their salt form the flesh and blood of their prey. This demand is necessity of/or all living for things for salt in one form or another continues today.Our bodies contain relatively large quantities of salt, as we may infer from the taste of “blood, sweat, and tears.”(Dalf.w.Knutmann, 1971) Salt, NaCl, is a chemical compound made of sodium and chloride which has been exceptionally important to humans for thousands of years, because it is one of the substances upon which all of life evolved to depend. Humans, like all life, need a supply of salt in order to simply survive. Salt's ability to preserve food was a foundation of civilization. It helped to eliminate the dependence on the seasonal availability of food and it allowed travel over long distances. However, salt was difficult to obtain, and so it was a highly valued trade item to the point of being considered a form of currency by certain peoples. Many salt roads, such as via salaria in Italy, had been established by the Bronze Age. -
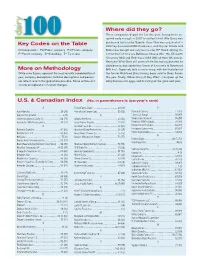
Key Codes on the Table More on Methodology Where Did They
Where did they go? Three companies depart the list this year, having been ac- quired early enough in 2007 to not be listed. Alto Dairy was Key Codes on the Table purchased last year by Saputo, Cass Clay was acquired at in 2007 by Associated Milk Producers, and Crystal Cream and C=Cooperative Pu=Public company Pr=Private company Butter was bought out early last year by HP Hood. Joining the P=Parent company S=Subsidiary T= Tie in rank list for the first time are BelGioso Cheese (No. 75), Ellsworth Creamery (84) and Roth Kase USA (96) all from Wisconsin. Next year Winn-Dixie will come off the list, having divested its dairy processing capabilities (some of it recently to Southeast More on Methodology Milk Inc.). Supervalu tells a similar story, with the final plant of While sales figures represent the most recently completed fiscal the former Richfood Dairy having been sold to Dean Foods year, company descriptions, facilities descriptions and person- this year. Finally, Wilcox Dairy of Roy, Wash., has given up the nel reflect recent changed where possible. Some entries will dairy business for eggs, and its listing will be gone next year. include an explanation of recent changes. U.S. & Canadian Index (No. in parentheses is last year’s rank) A Foster Farms Dairy ....................................... 50 (48) P Agri-Mark Inc. .............................................. 29 (29) Friendly Ice Cream Corp. ...............................55 (56) Parmalat Canada .........................................12 (13) Agropur Cooperative .........................................6 (9) G Perry’s Ice Cream ........................................ 97 (97) Anderson Erickson Dairy Co. ......................... 66 (71) Glanbia Foods Inc. ........................................ 23 (32) Plains Dairy Products ....................................95 (99) Associated Milk Producers Inc. -

Meats and Fish
ewteeetia9 Meats and Fish A. W. Oliver DATE. E. W. Harvey OF OUT IS information: PUBLICATIONcurrent mostFederal Cooperative Extension Service THIS Oregon State College For Corvallis Extension Bulletinhttp://extension.oregonstate.edu/catalog 731 December 1952 Cooperative Extension work in Agriculture and Home Economics, F. E. Price. director. Oregon State College and the United States Department of Agriculture cooperating. Printed and distributed in furtherance of Acts of Congress of May 8 and June 30, 1914. DATE. OF OUT IS information: PUBLICATIONcurrent most THIS For http://extension.oregonstate.edu/catalog NOTE: This bulletin is revised from and is to supersede Extension Bulletin 600, now out of print. 0#0teeffidief Meats and Fish By A. W. OLIVER, Associate Professor of Animal Husbandry, and E. W. HARVEY, Associate Food Technologist MEATS AND FISH are preserved mainly by salt. CURED\--) Other ingredients are added in some curing methods to give certain qualities to the products. Most cured meat and fish is smoked to aid in preserving and to add flavor. Two methods of curing are dry curing and brining. BriningDATE. is also called pickling. When sugar is added, the process is called dry sugar cure, or sweet pickling. Sweet pickling gives a moreuniform cure but can not be done at a temperature above OF40° F. Thedry sugar cure can be done at a temperature 400 to 45°F. The dry cure requires slightly less time in the cure but there is very little difference in the cured meat as to keeping time and other qualities. The method of curing beef is usually spokenOUT of as corning. -

Smoking Fish at Home
FNH-00325 UNIVERSITY OF ALASKA FAIRBANKS UNIVERSITY OF ALASKA FAIRBANKS Smoking Fish at Home Introduction much more easily than lean fish. Low-fat fish, such Producing high quality home-smoked fish is a as grayling and halibut, absorb salt quickly, and it is popular recreational activity and a point of pride easy to get the flesh too salty. for many Alaskans. In earlier times, smoking was Only high-quality fresh or frozen fish should be a form of food preservation; large amounts of salt used for smoking. Using poor-quality, freezer- and long smoking times were used to help pre- burned fish will produce a poor smoked product. serve the fish. Now fish is smoked more for flavor Smoking will not hide poor quality; it will call at- and appearance. Today’s lightly salted and smoked tention to the problem. fish is not a preserved product; the amounts of salt and smoke used are NOT sufficient to prevent When preparing fish for smoking, it is important to bacterial spoilage. Most food poisoning bacteria keep the preparation area clean and sanitary. Keep- can and will grow under the conditions normally ing the work area, cutting boards, knives and hands found in preparation and storage of smoked fish. clean will help prevent bacterial contamination. Do Clostridium botulinum, the bacteria that may cause not, under any circumstances, place your finished botulism, is the most harmful of these bacteria. smoked fish on unclean, unsanitized surfaces that have previously held raw fish. This could result in A safe, home-smoked product requires a good cross-contamination; spoilage bacteria could trans- understanding of the procedures and precautions fer from the raw to the finished product. -

Canal and Salt Town Middlewich, Cheshire Heritage Research
Canal and Salt Town Middlewich, Cheshire Heritage Research Report Middlewich Town Counci April 2009 SLR Ref: 406.1788.00002 406.1788.00002 Middlewich: Canal and Salt Town MIDDLEWICH CANAL AND SALT TOWN Introduction This report is one of the products of an exciting project started by Middlewich Town Council under their Middlewich Vision programme. The idea was to celebrate the salt and canal tradition of this historic town, and a successful bid to the Heritage Lottery Fund in 2007 allowed the Canal and Salt Town project to begin. The project included a canal boat festival and design of a trail along the canal, with interpretation boards to explain the history of the area. Research into the salt industry and canals, as well as gypsies and canal families at Middlewich resulted in information which was used for interpretation boards, an exhibition and a trail leaflet. It has also led to this report and archive that is available for educational purposes and for study by anyone who wishes to explore further, as well as a popular booklet which is a summary of the two aspects that has made Middlewich the place it is today. The sources used in this report are fully referenced at the end of each chapter. The report is presented in seven sections separated by blue dividers: 1 Salt 2 Canals 3 Gypsies 4 Geology 5 Flint Mill 6 Historic maps 7 Figures including photographs, reconstruction drawings and diagrams The report describes how the main focus for past communities at Middlewich was dominated by salt production. Salt was always important as a means to preserve food, and, as well as having a large number of other economic benefits, the uses that salt was put to by the people of Middlewich developed over the centuries into a highly sophisticated chemical industry. -

A Pest of Dairy Salts. Fred L
Volume 3 | Number 28 Article 8 July 2017 A pest of dairy salts. Fred L. Kent Iowa State College F. A. Leighton Iowa State College Follow this and additional works at: http://lib.dr.iastate.edu/bulletin Part of the Agriculture Commons, and the Dairy Science Commons Recommended Citation Kent, Fred L. and Leighton, F. A. (2017) "A pest of dairy salts.," Bulletin: Vol. 3 : No. 28 , Article 8. Available at: http://lib.dr.iastate.edu/bulletin/vol3/iss28/8 This Article is brought to you for free and open access by the Extension and Experiment Station Publications at Iowa State University Digital Repository. It has been accepted for inclusion in Bulletin by an authorized editor of Iowa State University Digital Repository. For more information, please contact [email protected]. Kent and Leighton: A234 pest of dairy salts. A. 'Pest of Dairy Salts. F r e d L. K e n t . F. A. L e i g h t o n . The claims of superiority set forth by the different manufacturers of dairy salts, led the Dairy Department of this station to make a test of several of the leading- brands of dairy salt with regard to their effect on the keeping qualities of butter. The matter of shrinkage, in work ing also came in incidentally. The butter for this test was churned Oct. 11th from the cream separated from 9316 pounds of milk, by the Danish Westsn and Alpha separators, The cream was ripened over night and put in the churns the next morning about 7:30. Each churn contained 80 gallons of about 25 per cent fat cream at a temperature of 53° ,■ free from taint or foreign odor and showing an acidity of 33 by Mann’s Acid Test. -

Preservation of Vegetables by Salting and Pickling by Winifred Jones, Specialist in Food Preservation Texas A
Issued by The Extension Service Agricultural and Mechanical College of Texas and The United States Department of Agriculture H. H. Williamson, Director, College Station, Texas 2 Preservation of Vegetables by Salting and Pickling by Winifred Jones, Specialist in Food Preservation Texas A. and M. College Extension Service Pickling is one of the oldest small amount of salt is used it known methods of food preser- does not have to be soaked vation. The preserving agents out. Therefore, the minerals in pickling are salt or vinegar, and most of the vitamins OT a combination of these. For found in the fresh vegetables flavor, spices mayor may not are in the kraut. be added. Pickling should only Method II. When a large supplement other methods of amount of dry salt is used, preservation such as canning, fermentation does not take freezing, and drying. Preser- place. The product is ready to vation of vegetables with seal and store on the second small amounts of salt is re- day. This method is recom- commended especially for mended only to those who families who do not have pres- have quantities of vegetables sure cookers, driers, or ade- and who do not have time, quate containers for canning equipment or material to pre- or drying. serve them by better methods. This bulletin is written in Salting with a large amount two parts--the first part is on of dry salt would be classed Salting and the second is on as the least desirable method Pi~kles and Relishes. of salting because a long soak- ing is necessary to remove the SALTING salt.