Chlorine Trifluoride
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

(VI) and Chromium (V) Oxide Fluorides
Portland State University PDXScholar Dissertations and Theses Dissertations and Theses 1976 The chemistry of chromium (VI) and chromium (V) oxide fluorides Patrick Jay Green Portland State University Follow this and additional works at: https://pdxscholar.library.pdx.edu/open_access_etds Part of the Chemistry Commons Let us know how access to this document benefits ou.y Recommended Citation Green, Patrick Jay, "The chemistry of chromium (VI) and chromium (V) oxide fluorides" (1976). Dissertations and Theses. Paper 4039. https://doi.org/10.15760/etd.5923 This Thesis is brought to you for free and open access. It has been accepted for inclusion in Dissertations and Theses by an authorized administrator of PDXScholar. Please contact us if we can make this document more accessible: [email protected]. All ABSTRACT OF THE TllESIS OF Patrick Jay Green for the Master of Science in Chemistry presented April 16, 1976. Title: Chemistry of Chromium(VI) and Chromium(V) Oxide Fluorides. APPROVEO BY MEMBERS OF THE THESIS CO'"o\l TIEE: y . • Ii . ' I : • • • • • New preparative routes to chromyl fluoride were sought. It was found that chlorine ironofluoride reacts with chromium trioxide and chromyl chlo ride to produce chromyl fluoride. Attempts were ~ade to define a mechan ism for the reaction of ClF and Cr0 in light of by-products observed 3 and previous investigations. Carbonyl fluoride and chromium trioxide react to fom chro·yl fluoride and carbo:i dioxide. A mechanism was also proposed for this react10n. Chromium trioxide 11itl\ l~F6 or WF5 reacts to produce chromyl fluoride and the respective oxide tetrafluoride. 2 Sulfur hexafluoride did not react with Cr03. -

2018 Annual Survey of Biological and Chemical Agents Regulated by Homeland Security (And Carcinogens Regulated by OSHA)
Name: Dept: Date: 2018 Annual Survey of Biological and Chemical Agents regulated by Homeland Security (and carcinogens regulated by OSHA) Due (date) All labs that do not have a current chemical inventory in Chematix MUST complete this survey. The University is required to make an annual report of all chemicals on the Chemical Facility Anti-Terrorism Standards (CFATS) lists. Additional information regarding the regulations is available on the EH&S website at http://www.safety.rochester.edu/restricted/occsafe/chemicalagent.html and https://www.selectagents.gov. 1. Please review the lists on the following pages and indicate if any are possessed by your lab. The CAS# has been added to the list for ease of searching databases. The CAS# is a Chemical Abstract Service numbering system which assigns a unique number to every chemical substance based on structure; this helps avoid confusion by use of synonyms or different naming conventions. a. If yes for possession, place an X in the applicable box and if requested, include the quantity held in your lab. b. If no, leave blank. 2. After reviewing the list, please complete the information box below (or on last page for possession), then sign, date and return to EH&S. 3. Please call Donna Douglass at 275-2402 if you have any questions. Thank you for your cooperation in collecting data required by the Department of Homeland Security! Possession: 1) Fill in applicable boxes, 2) have PI sign last page, 3) return all pages to Donna Douglass OR Non-possession: 1) Check only one box on the left, 2) sign, 3) return just this page to Donna Douglass I do not have a lab, do not work in a lab, nor do I possess any of the agents in this survey. -

Chlorine Trifluoride
MATERIAL SAFETY DATA SHEET Prepared to U.S. OSHA, CMA, ANSI and Canadian WHMIS Standards 1. PRODUCT IDENTIFICATION CHEMICAL NAME; CLASS: CHLORINE TRIFLUORIDE SYNONYMS: Chlorine Fluoride CHEMICAL FAMILY NAME: Halogen Fluoride FORMULA: ClF3 Document Number: 20026 PRODUCT USE: Use as a fluorinator; for cutting oil-well tubes; reprocessing reactor fuels, as an oxidizer in propellants. SUPPLIER/MANUFACTURER'S NAME: AIR LIQUIDE AMERICA CORPORATION ADDRESS: 2700 Post Oak Drive Houston, TX 77056-8229 EMERGENCY PHONE: CHEMTREC: 1-800-424-9300 BUSINESS PHONE: General MSDS Information 1-713/896-2896 Fax on Demand: 1-800/231-1366 2. COMPOSITION and INFORMATION ON INGREDIENTS CHEMICAL NAME CAS # mole % EXPOSURE LIMITS IN AIR ACGIH OSHA TLV STEL PEL STEL IDLH OTHER ppm ppm ppm ppm ppm Chlorine Trifluoride 7790-91-2 > 99% NE 0.1, C NE 0.1, C 20 NIOSH REL: 0.1 C ppm DFG MAK: 0.1 ppm, C Maximum Impurities < 1% None of the trace impurities in this product contribute significantly to the hazards associated with the product. All hazard information pertinent to this product has been provided in this Material Safety Data Sheet, per the requirements of the OSHA Hazard Communication Standard (29 CFR 1910.1200) and State equivalents standards. NE = Not Established C = Ceiling Limit See Section 16 for Definitions of Terms Used. NOTE: all WHMIS required information is included. It is located in appropriate sections based on the ANSI Z400.1-1993 format. CHLORINE TRIFLUORIDE - ClF3 MSDS EFFECTIVE DATE: JUNE 1, 1998 PAGE 1 OF 9 3. HAZARD IDENTIFICATION EMERGENCY OVERVIEW: Chlorine Trifluoride is an extremely toxic, corrosive, water-reactive, oxidizing, colorless, liquefied gas, with a suffocating, sweet odor. -

Aegls Brochure
4.85 5 5 About the Board on Environmental Studies and Toxicology The Board on Environmental Studies and Toxicology addresses Types of Chemicals Covered in the AEGLs Series environmental pollution problems affecting human health, human impacts on the environment, and the assessment and management of risks to AEGLs values for the chemicals listed below were published in the first human health and the environment. The board’s reports answer questions six volumes of the AEGLs series. AEGLs for additional chemicals will about air and water pollution; solid and hazardous waste; toxicology; continue to be published in subsequent volumes. epidemiology; risk assessment; applied ecology; natural resources; and environmental engineering, economics, law, and policy. Allylamine Hydrogen fluoride Ammonia Iron pentacarbonyl Aniline Methyl hydrazine Arsine Methyl isocyanate About NRC Reports from the National Academies Protecting Chlorine Nerve agents GA [tabun], The National Academies, through its National Research Council reports, Chlorine dioxide GB [sarin], GD [soman], GF, provides a unique public service by working outside the framework of Chlorine trifluoride and VX the Public and government to ensure independent, expert advice on matters of science, Crotonaldehyde Nickel carbonyl technology, and medicine. Today, the National Academies include three Cyclohexylamine Phosgene honorary societies that elect new members to their ranks each year- Diborane Phosphine Emergency the National Academy of Sciences, the National Academy of Engineering, 1,1-Dichloro-1-fluoroethane Propylene glycol dinitrate and the Institute of Medicine-and the National Research Council, the (HCFC-141B) Sulfur mustard operating arm that conducts the bulk of the institution’s Dimethylhydrazine 1,1,1,2-Tetrafluoroethane Workers science-policy and technical work. -

Chemical Chemical Hazard and Compatibility Information
Chemical Chemical Hazard and Compatibility Information Acetic Acid HAZARDS & STORAGE: Corrosive and combustible liquid. Serious health hazard. Reacts with oxidizing and alkali materials. Keep above freezing point (62 degrees F) to avoid rupture of carboys and glass containers.. INCOMPATIBILITIES: 2-amino-ethanol, Acetaldehyde, Acetic anhydride, Acids, Alcohol, Amines, 2-Amino-ethanol, Ammonia, Ammonium nitrate, 5-Azidotetrazole, Bases, Bromine pentafluoride, Caustics (strong), Chlorosulfonic acid, Chromic Acid, Chromium trioxide, Chlorine trifluoride, Ethylene imine, Ethylene glycol, Ethylene diamine, Hydrogen cyanide, Hydrogen peroxide, Hydrogen sulfide, Hydroxyl compounds, Ketones, Nitric Acid, Oleum, Oxidizers (strong), P(OCN)3, Perchloric acid, Permanganates, Peroxides, Phenols, Phosphorus isocyanate, Phosphorus trichloride, Potassium hydroxide, Potassium permanganate, Potassium-tert-butoxide, Sodium hydroxide, Sodium peroxide, Sulfuric acid, n-Xylene. Acetone HAZARDS & STORAGE: Store in a cool, dry, well ventilated place. INCOMPATIBILITIES: Acids, Bromine trifluoride, Bromine, Bromoform, Carbon, Chloroform, Chromium oxide, Chromium trioxide, Chromyl chloride, Dioxygen difluoride, Fluorine oxide, Hydrogen peroxide, 2-Methyl-1,2-butadiene, NaOBr, Nitric acid, Nitrosyl chloride, Nitrosyl perchlorate, Nitryl perchlorate, NOCl, Oxidizing materials, Permonosulfuric acid, Peroxomonosulfuric acid, Potassium-tert-butoxide, Sulfur dichloride, Sulfuric acid, thio-Diglycol, Thiotrithiazyl perchlorate, Trichloromelamine, 2,4,6-Trichloro-1,3,5-triazine -
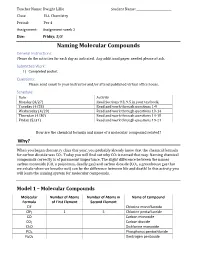
Naming Molecular Compounds General Instructions: Please Do the Activities for Each Day As Indicated
Teacher Name: Dwight Lillie Student Name: ________________________ Class: ELL Chemistry Period: Per 4 Assignment: Assignment week 2 Due: Friday, 5/8 Naming Molecular Compounds General Instructions: Please do the activities for each day as indicated. Any additional paper needed please attach. Submitted Work: 1) Completed packet. Questions: Please send email to your instructor and/or attend published virtual office hours. Schedule: Date Activity Monday (4/27) Read Sections 9.3, 9.5 in your textbook. Tuesday (4/28) Read and work through questions 1-9 Wednesday (4/29) Read and work through questions 10-14 Thursday (4/30) Read and work through questions 14-18 Friday (5/31) Read and work through questions 19-21 How are the chemical formula and name of a molecular compound related? Why? When you began chemistry class this year, you probably already knew that the chemical formula for carbon dioxide was CO2. Today you will find out why CO2 is named that way. Naming chemical compounds correctly is of paramount importance. The slight difference between the names carbon monoxide (CO, a poisonous, deadly gas) and carbon dioxide (CO2, a greenhouse gas that we exhale when we breathe out) can be the difference between life and death! In this activity you will learn the naming system for molecular compounds. Model 1 – Molecular Compounds Molecular Number of Atoms Number of Atoms in Name of Compound Formula of First Element Second Element ClF Chlorine monofluoride ClF5 1 5 Chlorine pentafluoride CO Carbon monoxide CO2 Carbon dioxide Cl2O Dichlorine monoxide PCl5 Phosphorus pentachloride N2O5 Dinitrogen pentoxide 1. Fill in the table to indicate the number of atoms of each type in the molecular formula. -

The Synthesis and Characterization of Novel Pentafluorosulfanyl-Containing Heterocycles and Pentafluorosulfanyldifluoromethane
Clemson University TigerPrints All Dissertations Dissertations May 2019 The yS nthesis and Characterization of Novel Pentafluorosulfanyl-Containing Heterocycles and Pentafluorosulfanyldifluoromethane Steven Paul Belina Clemson University, [email protected] Follow this and additional works at: https://tigerprints.clemson.edu/all_dissertations Recommended Citation Belina, Steven Paul, "The yS nthesis and Characterization of Novel Pentafluorosulfanyl-Containing Heterocycles and Pentafluorosulfanyldifluoromethane" (2019). All Dissertations. 2373. https://tigerprints.clemson.edu/all_dissertations/2373 This Dissertation is brought to you for free and open access by the Dissertations at TigerPrints. It has been accepted for inclusion in All Dissertations by an authorized administrator of TigerPrints. For more information, please contact [email protected]. THE SYNTHESIS AND CHARACTERIZATION OF NOVEL PENTAFLUOROSULFANYL-CONTAINING HETEROCYCLES AND PENTAFLUOROSULFANYLDIFLUOROMETHANE A Dissertation Presented to the Graduate School of Clemson University In Partial Fulfillment of the Requirements for the Degree Doctor of Philosophy Chemistry by Steven Paul Belina May 2019 Accepted by Joseph S. Thrasher, Ph.D., Committee Chair Julia L. Brumaghim, Ph.D. R. Karl Dieter, Ph.D. Joseph W. Kolis, Ph.D. Title Page ABSTRACT Beginning in the early 1960s, scientists began to experiment with the pentafluorosulfanyl moiety. The unique properties of the functional group has attracted interest among fluorine chemists and more recently even organic chemists. These properties include high group electronegativity, high steric bulk, high lipophilicity, a highly electron withdrawing nature, and a square pyramidal geometry. However, the development and deployment of the pentafluorosulfanyl functional group has been significantly slowed. The main cause for the slow development is a lack of easily available reagents to synthesize pentafluorosulfanyl-containing compounds. Historically, the primary reagents used for synthesizing pentafluorosulfanyl-containing compounds were SF5Cl and SF5Br. -

The WMS Model
Electronic Supplementary Material (ESI) for Physical Chemistry Chemical Physics. This journal is © the Owner Societies 2018 S-1 ELECTRONIC SUPPLEMENTARY INFORMATION OCT. 8, 2018 Extrapolation of High-Order Correlation Energies: The WMS Model Yan Zhao,a Lixue Xia,a Xiaobin Liao,a Qiu He,a Maria X. Zhao,b and Donald G. Truhlarc aState Key Laboratory of Silicate Materials for Architectures,International School of Materials Science and Engineering, Wuhan University of Technology, Wuhan 430070, People’s Republic of China. bLynbrook High School, 1280 Johnson Avenue, San Jose, California 95129 cDepartment of Chemistry and Supercomputing Institute, University of Minnesota, 207 Pleasant Street S.E., Minneapolis, MN 55455-0431 TABLE OF CONTENTS Table S1: Calculated classical barrier heights (kcal/mol) for the DBH24-W4 database S-2 Table S2: RMSE of the scalar relativistic contribution in WMS (kcal/mol) S-3 Table S3: RMSE of the composite methods for the W4-17 database (kcal/mol) S-4 Table S4: Calculated Born-Oppenheimer atomization energy (kcal/mol) for the W4-17 database S-5 Table S5: Example input file for a WMS calculation: the H2O molecule S-11 Table S6: Example input file for a WMS calculation: the CH3 radical S-12 Table S7. perl scripts for performing WMS calculations S-13 S-2 Table S1: Calculated classical barrier heights (kcal/mol) for the DBH24-W4 database (The ZPE contributions are excluded.) Reactions Best Est. WMS Hydrogen Atom Transfers ! ∆E! 6.35 6.25 OH + CH4 → CH3 + H2O ! ∆E! 19.26 19.28 ! ∆E! 10.77 10.88 H + OH → O + H2 ! ∆E! 13.17 -

Chlorine Trifluoride Ctf
CHLORINE TRIFLUORIDE CTF CAUTIONARY RESPONSE INFORMATION 4. FIRE HAZARDS 7. SHIPPING INFORMATION 4.1 Flash Point: 7.1 Grades of Purity: 99+% Common Synonyms Liquefied compressed Greenish yellow liquid Strong sweetish Not flammable, but may cause fire on 7.2 Storage Temperature: Ambient CTF gas or colorless gas odor contact with some materials. 7.3 Inert Atmosphere: No requirement 4.2 Flammable Limits in Air: Not pertinent 7.4 Venting: Safety relief Sinks and may boil in water. Reacts violently with water to produce 4.3 Fire Extinguishing Agents: Dry chemical 7.5 IMO Pollution Category: Currently not available poisonous gas. Boiling point is 53°F. 4.4 Fire Extinguishing Agents Not to Be Used: Do not use water on adjacent fires 7.6 Ship Type: Currently not available KEEP PEOPLE AWAY. AVOID CONTACT WITH LIQUID AND VAPOR. unless well protected against hydrogen 7.7 Barge Hull Type: Currently not available Avoid inhalation. fluoride gas. Wear chemical protective suit with self-contained breathing apparatus. 4.5 Special Hazards of Combustion Evacuate area in case of large discharge. Products: If released from container, 8. HAZARD CLASSIFICATIONS Call fire department. fumes are toxic and irritating. 8.1 49 CFR Category: Poison Gas Notify local health and pollution control agencies. 4.6 Behavior in Fire: If released from 8.2 49 CFR Class: 2.3 Protect water intakes. container, can increase the intensity of 8.3 49 CFR Package Group: Not pertinent. fire. Containers may explode. Not flammable. 8.4 Marine Pollutant: No Fire May explode on contact with combustibles. 4.7 Auto Ignition Temperature: Not pertinent 8.5 NFPA Hazard Classification: Not listed POISONOUS GASES ARE PRODUCED WHEN HEATED. -

Department of Homeland Security Chemicals of Interest
Department of Homeland Security Chemicals of Interest On October 4, 2006, President George W Bush signed the Homeland Security Appropriations Act of 2007. This Act gave the Department of Homeland Security (DHS) authority to regulate the security of facilities that contain high risk chemicals. On April 9, 2007, the Department of Homeland Security issued the Chemical Facility Anti- Terrorism Standards (CFATS). Under these standards the University must maintain an inventory of chemicals that are subject to this regulation. The chemicals subject to this regulation are more commonly known as chemicals of interest or COIs. In order to ensure the University of Northern Iowa remains compliant with this regulation, departments who come into possession of any of the chemicals listed on the following pages must immediately inform the Environmental Health and Safety (EH&S) Office of the name of the chemical or chemicals that were acquired, their respective amounts and storage location. Departments must also submit annual inventories of their chemicals to the EH&S office so an annual review of the amounts of COIs the University has on campus can be completed. If at any time it is found the amount of one or more of the COIs on campus exceeds the regulatory level (designated as threshold quantity by the DHS), the University must submit a report to the Department of Homeland Security (referred to as a “Top-Screen” by the DHS) within 60 days. Failure to report this information could result in fines to the University of $25,000 per day. The EH&S Office is responsible for gathering of campus chemical information and for submitting the Top Screen Report to the Department of Homeland Security on behalf of the University. -

United States Patent Office Patented Mar
3,373,000 United States Patent Office Patented Mar. 2, 1968 2 3,373,000 monofluoride is also an economical reactant, since it is PROCESS FOR PREPARING TETRAFLUORDES conveniently prepared by the disproportionation of ele AND HEXAFLUOR DES mental chlorine in liquid hydrogen fluoride, thereby avoid James J. Pitts, West Haven, and Albert W. Jache, North ing the use of expensive elemental fluorine. The selective Haven, Conn., assignors to Olin Mathieson Chemical fluorination process of this invention is particularly Sur Corporation, a corporation of Virginia prising and unexpected in view of the prior art which No Drawing. Filed Nov. 1, 1966, Ser. No. 591,079 teaches that chlorine monofluoride adds to a wide variety 9 Claims. (Cl. 23-352) of compounds, thereby acting as a chloro-fluorinating agent. For instance, U.S. Patent 3,035,893 discloses the 10 preparation of sulfur chloride pentafluoride from Sulfur ABSTRACT OF THE DISCLOSURE tetrafluoride and chlorine monofluoride. The tetrafluorides and hexafluorides of sulfur, selenium According to the process of this invention, the tetra and tellurium are provided by reacting chlorine mono fluorides and hexafluorides of sulfur, selenium and tel fluoride with sulfur, selenium, tellurium, metal sulfides, lurium are provided by reacting chlorine monofluoride metal selenides or metal tellurides. The hexafluorides of 15 with a material selected from the group consisting of Sul tungsten, molybdenum and uranium are provided by the fur, selenium, tellurium, metal sulfides, metal selenides reaction of chlorine monofluoride with the metals, their and metal tellurides. The hexafluorides of tungsten, oxides, sulfides and salts containing the metallate anion. molybdenum and uranium are provided by reacting These tetrafluorides and hexafluorides are well-known chlorine monofluoride with a material selected from the compounds, useful as fluorinating agents, gaseous di 20 group consisting of tungsten; molybdenum, uranium; the electrics, sources of high purity metallic powders, etc. -
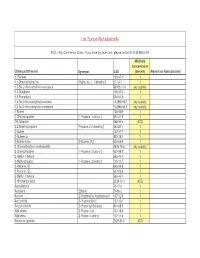
Homeland Security List
List Sorted Alphabetically ACG = Any Commercial Grade. If you have any questions, please contact EHS @ 898-5126. Minimum Concentration Chemical Of Interest Synonym CAS (percent) Amount on Hand (pounds) 1- Pentene 109-67-1 1 1,1-Dimethylhydrazine [Hydrazine, 1, 1-dimethyl-] 57-14-7 1 1,3-Bis(2-chloroethylthio)-n-propane 63905-10-2 any quantity 1,3-Butadiene 106-99-0 1 1,3-Pentadiene 504-60-9 1 1,4-Bis(2-chloroethylthio)-n-butane 142868-93-7 any quantity 1,5-Bis(2-chloroethylthio)-n-pentane 142868-94-8 any quantity 1-Butene 106-98-9 1 1-Chloropropylene [1-Propene, 1-chloro-] 590-21-6 1 1H-Tetrazole 288-94-8 ACG 2,2-Dimethylpropane [Propane, 2,2-dimethyl-] 463-82-1 1 2-Butene 107-01-7 1 2-Butene-cis 590-18-1 1 2-Butene-trans [2-Butene, (E)] 624-64-6 1 2-Chloroethylchloro-methylsulfide 2625-76-5 any quantity 2-Chloropropylene [1-Propene, 2-chloro-] 557-98-2 1 2-Methyl-1-butene 563-46-2 1 2-Methylpropene [1-Propene, 2-methyl-] 115-11-7 1 2-Pentene, (E)- 646-04-8 1 2-Pentene, (Z)- 627-20-3 1 3-Methyl-1-butene 563-45-1 1 5-Nitrobenzotriazol 2338-12-7 ACG Acetaldehyde 75-07-0 1 Acetylene [Ethyne] 74-86-2 1 Acrolein [2-Propenal] or Acrylaldehyde 107-02-8 1 Acrylonitrile [2-Propenenitrile] 107-13-1 1 Acrylyl chloride [2-Propenoyl chloride] 814-68-6 1 Allyl alcohol [2-Propen-1-ol] 107-18-6 1 Allylamine [2-Propen-1-amine] 107-11-9 1 Aluminum (powder) 7429-90-5 ACG Ammonia (anhydrous) 7664-41-7 1 Ammonia (conc.