Chlorine Trifluoride
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

(VI) and Chromium (V) Oxide Fluorides
Portland State University PDXScholar Dissertations and Theses Dissertations and Theses 1976 The chemistry of chromium (VI) and chromium (V) oxide fluorides Patrick Jay Green Portland State University Follow this and additional works at: https://pdxscholar.library.pdx.edu/open_access_etds Part of the Chemistry Commons Let us know how access to this document benefits ou.y Recommended Citation Green, Patrick Jay, "The chemistry of chromium (VI) and chromium (V) oxide fluorides" (1976). Dissertations and Theses. Paper 4039. https://doi.org/10.15760/etd.5923 This Thesis is brought to you for free and open access. It has been accepted for inclusion in Dissertations and Theses by an authorized administrator of PDXScholar. Please contact us if we can make this document more accessible: [email protected]. All ABSTRACT OF THE TllESIS OF Patrick Jay Green for the Master of Science in Chemistry presented April 16, 1976. Title: Chemistry of Chromium(VI) and Chromium(V) Oxide Fluorides. APPROVEO BY MEMBERS OF THE THESIS CO'"o\l TIEE: y . • Ii . ' I : • • • • • New preparative routes to chromyl fluoride were sought. It was found that chlorine ironofluoride reacts with chromium trioxide and chromyl chlo ride to produce chromyl fluoride. Attempts were ~ade to define a mechan ism for the reaction of ClF and Cr0 in light of by-products observed 3 and previous investigations. Carbonyl fluoride and chromium trioxide react to fom chro·yl fluoride and carbo:i dioxide. A mechanism was also proposed for this react10n. Chromium trioxide 11itl\ l~F6 or WF5 reacts to produce chromyl fluoride and the respective oxide tetrafluoride. 2 Sulfur hexafluoride did not react with Cr03. -

Chemical Chemical Hazard and Compatibility Information
Chemical Chemical Hazard and Compatibility Information Acetic Acid HAZARDS & STORAGE: Corrosive and combustible liquid. Serious health hazard. Reacts with oxidizing and alkali materials. Keep above freezing point (62 degrees F) to avoid rupture of carboys and glass containers.. INCOMPATIBILITIES: 2-amino-ethanol, Acetaldehyde, Acetic anhydride, Acids, Alcohol, Amines, 2-Amino-ethanol, Ammonia, Ammonium nitrate, 5-Azidotetrazole, Bases, Bromine pentafluoride, Caustics (strong), Chlorosulfonic acid, Chromic Acid, Chromium trioxide, Chlorine trifluoride, Ethylene imine, Ethylene glycol, Ethylene diamine, Hydrogen cyanide, Hydrogen peroxide, Hydrogen sulfide, Hydroxyl compounds, Ketones, Nitric Acid, Oleum, Oxidizers (strong), P(OCN)3, Perchloric acid, Permanganates, Peroxides, Phenols, Phosphorus isocyanate, Phosphorus trichloride, Potassium hydroxide, Potassium permanganate, Potassium-tert-butoxide, Sodium hydroxide, Sodium peroxide, Sulfuric acid, n-Xylene. Acetone HAZARDS & STORAGE: Store in a cool, dry, well ventilated place. INCOMPATIBILITIES: Acids, Bromine trifluoride, Bromine, Bromoform, Carbon, Chloroform, Chromium oxide, Chromium trioxide, Chromyl chloride, Dioxygen difluoride, Fluorine oxide, Hydrogen peroxide, 2-Methyl-1,2-butadiene, NaOBr, Nitric acid, Nitrosyl chloride, Nitrosyl perchlorate, Nitryl perchlorate, NOCl, Oxidizing materials, Permonosulfuric acid, Peroxomonosulfuric acid, Potassium-tert-butoxide, Sulfur dichloride, Sulfuric acid, thio-Diglycol, Thiotrithiazyl perchlorate, Trichloromelamine, 2,4,6-Trichloro-1,3,5-triazine -

Chemical Names and CAS Numbers Final
Chemical Abstract Chemical Formula Chemical Name Service (CAS) Number C3H8O 1‐propanol C4H7BrO2 2‐bromobutyric acid 80‐58‐0 GeH3COOH 2‐germaacetic acid C4H10 2‐methylpropane 75‐28‐5 C3H8O 2‐propanol 67‐63‐0 C6H10O3 4‐acetylbutyric acid 448671 C4H7BrO2 4‐bromobutyric acid 2623‐87‐2 CH3CHO acetaldehyde CH3CONH2 acetamide C8H9NO2 acetaminophen 103‐90‐2 − C2H3O2 acetate ion − CH3COO acetate ion C2H4O2 acetic acid 64‐19‐7 CH3COOH acetic acid (CH3)2CO acetone CH3COCl acetyl chloride C2H2 acetylene 74‐86‐2 HCCH acetylene C9H8O4 acetylsalicylic acid 50‐78‐2 H2C(CH)CN acrylonitrile C3H7NO2 Ala C3H7NO2 alanine 56‐41‐7 NaAlSi3O3 albite AlSb aluminium antimonide 25152‐52‐7 AlAs aluminium arsenide 22831‐42‐1 AlBO2 aluminium borate 61279‐70‐7 AlBO aluminium boron oxide 12041‐48‐4 AlBr3 aluminium bromide 7727‐15‐3 AlBr3•6H2O aluminium bromide hexahydrate 2149397 AlCl4Cs aluminium caesium tetrachloride 17992‐03‐9 AlCl3 aluminium chloride (anhydrous) 7446‐70‐0 AlCl3•6H2O aluminium chloride hexahydrate 7784‐13‐6 AlClO aluminium chloride oxide 13596‐11‐7 AlB2 aluminium diboride 12041‐50‐8 AlF2 aluminium difluoride 13569‐23‐8 AlF2O aluminium difluoride oxide 38344‐66‐0 AlB12 aluminium dodecaboride 12041‐54‐2 Al2F6 aluminium fluoride 17949‐86‐9 AlF3 aluminium fluoride 7784‐18‐1 Al(CHO2)3 aluminium formate 7360‐53‐4 1 of 75 Chemical Abstract Chemical Formula Chemical Name Service (CAS) Number Al(OH)3 aluminium hydroxide 21645‐51‐2 Al2I6 aluminium iodide 18898‐35‐6 AlI3 aluminium iodide 7784‐23‐8 AlBr aluminium monobromide 22359‐97‐3 AlCl aluminium monochloride -

Perchloryl Fluoride Final AEGL
This PDF is available from The National Academies Press at http://www.nap.edu/catalog.php?record_id=15852 Acute Exposure Guideline Levels for Selected Airborne Chemicals: Volume 13 ISBN Committee on Acute Exposure Guideline Levels; Committee on 978-0-309-29025-8 Toxicology; Board on Environmental Studies and Toxicology; Division on Earth and Life Studies; National Research Council 292 pages 6 x 9 PAPERBACK (2013) Visit the National Academies Press online and register for... Instant access to free PDF downloads of titles from the NATIONAL ACADEMY OF SCIENCES NATIONAL ACADEMY OF ENGINEERING INSTITUTE OF MEDICINE NATIONAL RESEARCH COUNCIL 10% off print titles Custom notification of new releases in your field of interest Special offers and discounts Distribution, posting, or copying of this PDF is strictly prohibited without written permission of the National Academies Press. Unless otherwise indicated, all materials in this PDF are copyrighted by the National Academy of Sciences. Request reprint permission for this book Copyright © National Academy of Sciences. All rights reserved. Acute Exposure Guideline Levels for Selected Airborne Chemicals: Volume 13 Committee on Acute Exposure Guideline Levels Committee on Toxicology Board on Environmental Studies and Toxicology Division on Earth and Life Studies Copyright © National Academy of Sciences. All rights reserved. Acute Exposure Guideline Levels for Selected Airborne Chemicals: Volume 13 THE NATIONAL ACADEMIES PRESS 500 FIFTH STREET, NW WASHINGTON, DC 20001 NOTICE: The project that is the subject of this report was approved by the Governing Board of the National Research Council, whose members are drawn from the councils of the National Academy of Sciences, the National Academy of Engineering, and the Insti- tute of Medicine. -
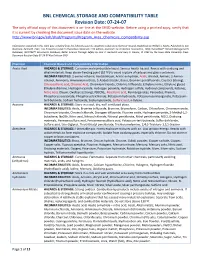
BNL CHEMICAL STORAGE and COMPATIBILITY TABLE Revision Date: 07-24-07 the Only Official Copy of This Document Is On-Line at the SHSD Website
BNL CHEMICAL STORAGE AND COMPATIBILITY TABLE Revision Date: 07-24-07 The only official copy of this document is on-line at the SHSD website. Before using a printed copy, verify that it is current by checking the document issue date on the website. http://www.bnl.gov/esh/shsd/Programs/Program_Area_Chemicals_Compatibility.asp Information contained in this table was compiled from the following sources: Academic Laboratory Chemical Hazards Guidebook by William J. Mahn, Published by Van Nostrand, Reinhold, 1991; Fire Protection Guide to Hazardous Materials 11th edition, National Fire Protection Association, 1994; Hazardtext® Hazard Managements Database; INFOTEXT® Documents Database; Better Science Through Safety by Jack A. Gerlovich and Gary E. Downs, © 1981 by the Iowa State University Press. Document Revision Date 07-24-07 Ken Erickson CHO Chemical Chemical Hazard and Compatibility Information Acetic Acid HAZARDS & STORAGE: Corrosive and combustible liquid. Serious health hazard. Reacts with oxidizing and alkali materials. Keep above freezing point (62 ºF) to avoid rupture of carboys and glass containers. INCOMPATIBILITIES: 2-amino-ethanol, Acetaldehyde, Acetic anhydride, Acids, Alcohol, Amines, 2-Amino- ethanol, Ammonia, Ammonium nitrate, 5-Azidotetrazole, Bases, Bromine pentafluoride, Caustics (strong), Chlorosulfonic acid, Chromic Acid, Chromium trioxide, Chlorine trifluoride, Ethylene imine, Ethylene glycol, Ethylene diamine, Hydrogen cyanide, Hydrogen peroxide, Hydrogen sulfide, Hydroxyl compounds, Ketones, Nitric Acid, Oleum, Oxidizers -

Unit 10 Elements of Group 17
UNIT 10 ELEMENTS OF GROUP 17 Introduction Objectives Occurrence, Extraction and Uses (xxlmma Exlraction Preparation of Fluorine uses General Characteristics Physical Properties Oxidation States Oxidising Power Chemical Properties Basic Properties of Halogens Compounds of Halogens Hydmgd Halides Halogen Oxides Oxoacids of Halogens Interhalogen Compounds Polyhalides and Polyhalonium Ions 1 Pseudohalogens and Pseudohalides ~nomalous~~ehaviourof Fluorine Summary Terminal Questions Answers 10.1 INTRODUCTION In Unit 1 you studied the.periodic table which gives the classification of elements into various periods and groups. You have seen that in any period the element in Group 1 is the most electropositive and metallic in nature. As we go across a period from Group 1. to Group 17 of the main group elements, nonmetallic nature, ionisation energy, electron affinityand elecmnegativity increase, reaching a maximum at Group 17. In this way, at one extreme we have Group 1 comprising alkali metals and at the other we have Group 17 comprising non- metals, namely, fluorine, chlorine, bromine, iodine and astatine, collectively called the halogens. Halogens derive their name from the Greek words, halos + gens meaning salt producers, as they form salts in combination with metals. The most common of the salts being sodium chloride or the common salt. Halogens find a wide variety of uses in everyday life. In view of their nature and usefulness, it will be interesting to study the chemistry of halogens. After studying this unit, you should be able to: explain the occurrence, extraction and uses of halogens, describe the isolation of fluorine, list the general characteristics of halogens and describe their reactions, describe the chemistry of hydrogen halihes, halogen oxides and oxoacids, describe the chemistry and geometry of interhalogen compounds and polyhalides, and explah the anomalous behaviour of fluorine. -

Chlorine Trifluoride
Chlorine trifluoride (CAS reg no: 7790-91-2) Health-based Reassessment of Administrative Occupational Exposure Limits Committee on Updating of Occupational Exposure Limits, a committee of the Health Council of the Netherlands No. 2000/15OSH/019, The Hague, 13 November 2001 019-1 Preferred citation: Health Council of the Netherlands: Committee on Updating of Occupational Exposure Limits. Chlorine trifluoride; Health-based Reassessment of Administrative Occupational Exposure Limits. The Hague: Health Council of the Netherlands, 2001; 2000/15OSH/019. all rights reserved 019-2 1 Introduction The present document contains the assessment of the health hazard of chlorine trifluoride by the Committee on Updating of Occupational Exposure Limits, a committee of the Health Council of the Netherlands. The first draft of this document was prepared by A. Wientjes, M.Sc. and H. Stouten, M.Sc. (TNO Nutrition and Food Research, Zeist, the Netherlands). The evaluation of the toxicity of chlorine trifluoride has been based on the review by the American Conference of Governmental Industrial Hygienists (ACG99). Where relevant, the original publications were reviewed and evaluated as will be indicated in the text. In addition, literature was retrieved from the online data bases Medline, Toxline, and Chemical Abstracts covering the period 1966 to 26 April 1999 (19990426/UP), 1965 to 29 January 1999 (19990129/ED), and 1967 to April 24 1999 (19990424/ED), respectively, using the following key words: chlorine trifluoride, chlorine fluoride, chlorotrifluoride, trifluorochlorine, ClF3, Cl2F6, and 7790-91-2. HSDB and RTECS, data bases available from CD-ROM, were consulted as well (NIO99, NLM99). The final literature search has been carried out in April 1999. -

Contents 11/29 Molecular Constants
Contents 11/29 Molecular Constants Subvolume D3 1 General Introduction 1 1.1 General remarks 1 1.2 Review articles and tables 1 1.3 Arrangement of tables, substances and parameters 1 1.4 Error notation 2 1.5 Selection of data 3 1.6 Abbreviations used for experimental methods 3 1.7 Selected fundamental constants and conversion factors 3 1.8 References for 1 5 2 Asymmetric Top Molecules: Introduction 6 2.1 Rotational parameters 6 2.1.1 Defining equations 6 2.1.2 List of tabulated rotational parameters 10 2.1.3 References for 2.1 12 2.2. Hyperfine coupling constants 13 2.2.1 Quadrupole coupling constants, defining equations 13 2.2.2 Magnetic-interaction constants, defining equations 15 2.2.3 List of tabulated asymmetric-top hfs parameters 20 2.2.4 References for 2.2 21 2.3 Internal rotation 23 2.3.1 Defining equations 23 2.3.2 List of tabulated internal-rotation parameters 26 2.3.3 Conversion factors 28 2.3.4 References for 2.3 28 2.4 Symmetric top electric dipole moments 29 2.4.1 References for 2.4 29 2.5 External field magnetic interaction parameters 30 2.5.1 Defining equations 30 2.5.2 List of tabulated asymmetric-top external-magnetic-field parameters 30 2.5.3 References for 2.5 30 3 Data (J. DEMAISON, J. VOGT) 31 581 C6HN 2-(Cyanoethynyl)-2-cyclopropen-l-ylidene 31 582 C6H2 l,2,3-Hexatrien-5-yn-l-ylidene 32 583 C6H2 1,2,3,4,5-Hexapentaenylidene 33 584 C6H2S 1,2,3,4,5-Hexapentaene-l-thione 34 585 C6H3ArF3 1,2,3-Trifluorobenzene-argon (1/1) 35 586 C6H4 (3Z)-3-Hexene-l,5-diyne 36 587 C6H4 l-Hexene-3,5-diyne 38 588 C6H4 l,3-Cydohexadien-5-yne -

United States Patent (19 3,876,754 Pursley I45 Apr
United States Patent (19 3,876,754 Pursley i45 Apr. 8, 1975 54 PREPARATION OF CHORINE PENTAFUORDE EXEMPLARY CAM (75 Inventor: John A. Pursley, Northridge, Calif. 1. A process for producing chlorine pentafluoride (73) Assignee: North American Rockwell comprising the steps of continuously passing fluorinc Corporation as one reactant and at least one member of the group consisting of chlorine, chlorine monofluoride and 22 Filed: June 9, 1964 chlorine trifluoride as another reactant into a reaction 21 Appl. No.: 374,886 chamber adjacent the lower end of the chamber, maintaining the temperature and pressure in the reac tion chamber sufficiently high to effect reaction be 52 U.S. Ct...................................... 423/466; 1491 tween sail reactants to form gaseous chlorine penta 5 l l int. Cl........................... C01b 7/24: CO 1 h 9) 8 fluoride in a mixture with gaseous fluorine; continu 58 Field of Search........................ 23/2()5; 423f466 ously passing said mixture from the reaction cham her at a place adjacent the upper end of the chamber into 56. References Cited a condensing chamber at a place adjacent the upper UNITED STATES PATENIS end of the condensing chamber; maintaining the tem 2,982,62() 51196 Beattie et al.......................... 23/205 perature in said condensing chamber sufficiently low 3.34.638 5/1964 Lawton et al..................... 23/205 X to effect condensation of chlorine pentafluoride from OTHER PUBLICATIONS said mixture; withdrawing condensed chlorine penta Stacey et al., Advances in Fluorine Chemistry, Vol. 4, fluoride from said condensing chamber, and continu Butterworths Inc., Washington, D.C., 1965, pp. 240 to (usly recirculating the uncondensed gils from the con 242. -

105 Cmr: Department of Public Health
105 CMR: DEPARTMENT OF PUBLIC HEALTH 105 CMR 670.000: "RIGHT TO KNOW" Section 670.001: Purpose 670.005: Definitions 670.010: The Massachusetts Substance List 670.020: Trade Secrets 670.025: Physician's Access to Material Safety Data Sheets Appendix A 670.001: Purpose The purpose of 105 CMR 670.000 is to protect the public health by providing and encouraging the greatest possible transmission of health and safety information concerning toxic and hazardous substances. 670.005: Definitions As used in 105 CMR 670.000 the following words and phrases have the following meanings: Carcinogen means: (1) any substance or combination of substances which causes an increased incidence of benign and/or malignant neoplasms in one or more species; or, (2) any substance or combination of substances which substantially decreases the latency period between exposure and onset of a neoplasm in one or more species; or, (3) any substance or combination of substances which is metabolized into one or more substances that causes an increased incidence of neoplasms or decreases the latency period between exposure and onset of neoplasms in one or more species. CAS Number means the identification number assigned by the Chemical Abstracts Service to a specific chemical substance. Chemical Name means the scientific designation of a substance in accordance with the nomenclature system developed by the International Union of Pure and Applied Chemistry, or the system developed by the Chemical Abstracts Service. Commissioner means the Commissioner of Public Health. Common Name means any designation or identification such as a code name, code number, trade name, or brand name used to identify a substance other than by its chemical name. -

Vapor Pressure 6-60
VAPOR PRESSURE This table gives vapor pressure data for about 1800 inorganic and organic substances. In order to accommodate elements and compounds ranging from refractory to highly volatile in a single table, the temperature at which the vapor pressure reaches specified pressure values is listed. The pressure values run in decade steps from 1 Pa (about 7.5 µm Hg) to 100 kPa (about 750 mm Hg). All temperatures are given in °C. The data used in preparing the table came from a large number of sources; the main references used for each substance are indicated in the last column. Since the data were refit in most cases, values appearing in this table may not be identical with values in the source cited. The temperature entry in the 100 kPa column is close to, but not identical with, the normal boiling point (which is defined as the temperature at which the vapor pressure reaches 101.325 kPa). Although some temperatures are quoted to 0.1°C, uncertainties of several degrees should generally be assumed. Values followed by an “e” were obtained by extrapolating (usually with an Antoine equation) beyond the region for which experimental measurements were available and are thus subject to even greater uncertainty. Compounds are listed by molecular formula following the Hill convention. Substances not containing carbon are listed first, followed by those that contain carbon. To locate an organic compound by name or CAS Registry Number when the molecular formula is not known, use the table Physical Constants of Organic Compounds in Section 3 and its indexes to determine the molecular formula. -

Chlorine Trifluoride Final AEGL Document
Committee on Acute Exposure Guideline Levels Committee on Toxicology Board on Environmental Studies and Toxicology Division on Earth and Life Studies THE NATIONAL ACADEMIES PRESS 500 Fifth Street, NW Washington, DC 20001 NOTICE: The project that is the subject of this report was approved by the Governing Board of the National Research Council, whose members are drawn from the councils of the National Academy of Sciences, the National Academy of Engineering, and the Insti- tute of Medicine. The members of the committee responsible for the report were chosen for their special competences and with regard for ap¬propriate balance. This project was supported by Contract No. DAMD17-99-C-9049 between the National Academy of Sciences and the U.S. Department of Defense and Contract No. 68-C-03-081 between the National Academy of Sciences and the U.S. Environmental Protection Agency. Any opinions, findings, conclusions, or recommendations expressed in this publication are those of the author(s) and do not necessarily reflect the view of the or- ganizations or agencies that provided support for this project. International Standard Book Number-13: 978-0-309-10358-9 International Standard Book Number-10: 0-309-10358-4 Additional copies of this report are available from The National Academies Press 500 Fifth Street, NW Box 285 Washington, DC 20055 800-624-6242 202-334-3313 (in the Washington metropolitan area) http://www.nap.edu Copyright 2007 by the National Academy of Sciences. All rights reserved. Printed in the United States of America The National Academy of Sciences is a private, nonprofit, self-perpetuating society of distinguished scholars engaged in scientific and engineering research, dedicated to the furtherance of science and technology and to their use for the general welfare.