(IDLH) Value Profile Bromine Trifluoride
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Transport of Dangerous Goods
ST/SG/AC.10/1/Rev.16 (Vol.I) Recommendations on the TRANSPORT OF DANGEROUS GOODS Model Regulations Volume I Sixteenth revised edition UNITED NATIONS New York and Geneva, 2009 NOTE The designations employed and the presentation of the material in this publication do not imply the expression of any opinion whatsoever on the part of the Secretariat of the United Nations concerning the legal status of any country, territory, city or area, or of its authorities, or concerning the delimitation of its frontiers or boundaries. ST/SG/AC.10/1/Rev.16 (Vol.I) Copyright © United Nations, 2009 All rights reserved. No part of this publication may, for sales purposes, be reproduced, stored in a retrieval system or transmitted in any form or by any means, electronic, electrostatic, magnetic tape, mechanical, photocopying or otherwise, without prior permission in writing from the United Nations. UNITED NATIONS Sales No. E.09.VIII.2 ISBN 978-92-1-139136-7 (complete set of two volumes) ISSN 1014-5753 Volumes I and II not to be sold separately FOREWORD The Recommendations on the Transport of Dangerous Goods are addressed to governments and to the international organizations concerned with safety in the transport of dangerous goods. The first version, prepared by the United Nations Economic and Social Council's Committee of Experts on the Transport of Dangerous Goods, was published in 1956 (ST/ECA/43-E/CN.2/170). In response to developments in technology and the changing needs of users, they have been regularly amended and updated at succeeding sessions of the Committee of Experts pursuant to Resolution 645 G (XXIII) of 26 April 1957 of the Economic and Social Council and subsequent resolutions. -
![Immediately Dangerous to Life Or Health (Idlh) Value Profile for Chlorine Pentafluoride [Cas No. 13637-63-3] and Bromine Pentafl](https://docslib.b-cdn.net/cover/6027/immediately-dangerous-to-life-or-health-idlh-value-profile-for-chlorine-pentafluoride-cas-no-13637-63-3-and-bromine-pentafl-116027.webp)
Immediately Dangerous to Life Or Health (Idlh) Value Profile for Chlorine Pentafluoride [Cas No. 13637-63-3] and Bromine Pentafl
External Review Draft March 2015 1 2 3 4 5 6 7 IMMEDIATELY DANGEROUS TO LIFE OR HEALTH (IDLH) VALUE PROFILE 8 9 10 11 FOR 12 13 14 15 CHLORINE PENTAFLUORIDE [CAS NO. 13637-63-3] 16 17 AND 18 19 BROMINE PENTAFLUORIDE [CAS NO. 7789-30-2] 20 21 22 23 24 25 26 Department of Health and Human Services 27 Centers for Disease Control and Prevention 28 National Institute for Occupational Safety and Health This information is distributed solely for the purpose of pre-dissemination peer review under applicable information quality guidelines. It has not been formally disseminated by the National Institute for Occupational Safety and Health. It does not represent and should not be construed to represent any agency determination or policy. i External Review Draft March 2015 1 DISCLAIMER 2 Mention of any company or product does not constitute endorsement by the National Institute for Occupational 3 Safety and Health (NIOSH). In addition, citations of Web sites external to NIOSH do not constitute NIOSH 4 endorsement of the sponsoring organizations or their programs or products. Furthermore, NIOSH is not 5 responsible for the content of these Web sites. 6 7 ORDERING INFORMATION 8 This document is in the public domain and may be freely copied or reprinted. To receive NIOSH documents or 9 other information about occupational safety and health topics, contact NIOSH at 10 Telephone: 1-800-CDC-INFO (1-800-232-4636) 11 TTY: 1-888-232-6348 12 E-mail: [email protected] 13 14 or visit the NIOSH Web site at www.cdc.gov/niosh. -

(VI) and Chromium (V) Oxide Fluorides
Portland State University PDXScholar Dissertations and Theses Dissertations and Theses 1976 The chemistry of chromium (VI) and chromium (V) oxide fluorides Patrick Jay Green Portland State University Follow this and additional works at: https://pdxscholar.library.pdx.edu/open_access_etds Part of the Chemistry Commons Let us know how access to this document benefits ou.y Recommended Citation Green, Patrick Jay, "The chemistry of chromium (VI) and chromium (V) oxide fluorides" (1976). Dissertations and Theses. Paper 4039. https://doi.org/10.15760/etd.5923 This Thesis is brought to you for free and open access. It has been accepted for inclusion in Dissertations and Theses by an authorized administrator of PDXScholar. Please contact us if we can make this document more accessible: [email protected]. All ABSTRACT OF THE TllESIS OF Patrick Jay Green for the Master of Science in Chemistry presented April 16, 1976. Title: Chemistry of Chromium(VI) and Chromium(V) Oxide Fluorides. APPROVEO BY MEMBERS OF THE THESIS CO'"o\l TIEE: y . • Ii . ' I : • • • • • New preparative routes to chromyl fluoride were sought. It was found that chlorine ironofluoride reacts with chromium trioxide and chromyl chlo ride to produce chromyl fluoride. Attempts were ~ade to define a mechan ism for the reaction of ClF and Cr0 in light of by-products observed 3 and previous investigations. Carbonyl fluoride and chromium trioxide react to fom chro·yl fluoride and carbo:i dioxide. A mechanism was also proposed for this react10n. Chromium trioxide 11itl\ l~F6 or WF5 reacts to produce chromyl fluoride and the respective oxide tetrafluoride. 2 Sulfur hexafluoride did not react with Cr03. -

Chlorine Trifluoride
MATERIAL SAFETY DATA SHEET Prepared to U.S. OSHA, CMA, ANSI and Canadian WHMIS Standards 1. PRODUCT IDENTIFICATION CHEMICAL NAME; CLASS: CHLORINE TRIFLUORIDE SYNONYMS: Chlorine Fluoride CHEMICAL FAMILY NAME: Halogen Fluoride FORMULA: ClF3 Document Number: 20026 PRODUCT USE: Use as a fluorinator; for cutting oil-well tubes; reprocessing reactor fuels, as an oxidizer in propellants. SUPPLIER/MANUFACTURER'S NAME: AIR LIQUIDE AMERICA CORPORATION ADDRESS: 2700 Post Oak Drive Houston, TX 77056-8229 EMERGENCY PHONE: CHEMTREC: 1-800-424-9300 BUSINESS PHONE: General MSDS Information 1-713/896-2896 Fax on Demand: 1-800/231-1366 2. COMPOSITION and INFORMATION ON INGREDIENTS CHEMICAL NAME CAS # mole % EXPOSURE LIMITS IN AIR ACGIH OSHA TLV STEL PEL STEL IDLH OTHER ppm ppm ppm ppm ppm Chlorine Trifluoride 7790-91-2 > 99% NE 0.1, C NE 0.1, C 20 NIOSH REL: 0.1 C ppm DFG MAK: 0.1 ppm, C Maximum Impurities < 1% None of the trace impurities in this product contribute significantly to the hazards associated with the product. All hazard information pertinent to this product has been provided in this Material Safety Data Sheet, per the requirements of the OSHA Hazard Communication Standard (29 CFR 1910.1200) and State equivalents standards. NE = Not Established C = Ceiling Limit See Section 16 for Definitions of Terms Used. NOTE: all WHMIS required information is included. It is located in appropriate sections based on the ANSI Z400.1-1993 format. CHLORINE TRIFLUORIDE - ClF3 MSDS EFFECTIVE DATE: JUNE 1, 1998 PAGE 1 OF 9 3. HAZARD IDENTIFICATION EMERGENCY OVERVIEW: Chlorine Trifluoride is an extremely toxic, corrosive, water-reactive, oxidizing, colorless, liquefied gas, with a suffocating, sweet odor. -

Assessment of Portable HAZMAT Sensors for First Responders
The author(s) shown below used Federal funds provided by the U.S. Department of Justice and prepared the following final report: Document Title: Assessment of Portable HAZMAT Sensors for First Responders Author(s): Chad Huffman, Ph.D., Lars Ericson, Ph.D. Document No.: 246708 Date Received: May 2014 Award Number: 2010-IJ-CX-K024 This report has not been published by the U.S. Department of Justice. To provide better customer service, NCJRS has made this Federally- funded grant report available electronically. Opinions or points of view expressed are those of the author(s) and do not necessarily reflect the official position or policies of the U.S. Department of Justice. Assessment of Portable HAZMAT Sensors for First Responders DOJ Office of Justice Programs National Institute of Justice Sensor, Surveillance, and Biometric Technologies (SSBT) Center of Excellence (CoE) March 1, 2012 Submitted by ManTech Advanced Systems International 1000 Technology Drive, Suite 3310 Fairmont, West Virginia 26554 Telephone: (304) 368-4120 Fax: (304) 366-8096 Dr. Chad Huffman, Senior Scientist Dr. Lars Ericson, Director UNCLASSIFIED This project was supported by Award No. 2010-IJ-CX-K024, awarded by the National Institute of Justice, Office of Justice Programs, U.S. Department of Justice. The opinions, findings, and conclusions or recommendations expressed in this publication are those of the author(s) and do not necessarily reflect those of the Department of Justice. This document is a research report submitted to the U.S. Department of Justice. This report has not been published by the Department. Opinions or points of view expressed are those of the author(s) and do not necessarily reflect the official position or policies of the U.S. -

Safety Data Sheet According to 29CFR1910/1200 and GHS Rev
Safety Data Sheet according to 29CFR1910/1200 and GHS Rev. 3 Effective date : 01.06.2015 Page 1 of 7 Methylene Blue, Loeffler's SECTION 1 : Identification of the substance/mixture and of the supplier Product name : Methylene Blue, Loeffler's Manufacturer/Supplier Trade name: Manufacturer/Supplier Article number: S25432 Recommended uses of the product and uses restrictions on use: Manufacturer Details: AquaPhoenix Scientific 9 Barnhart Drive, Hanover, PA 17331 Supplier Details: Fisher Science Education 15 Jet View Drive, Rochester, NY 14624 Emergency telephone number: Fisher Science Education Emergency Telephone No.: 800-535-5053 SECTION 2 : Hazards identification Classification of the substance or mixture: Flammable Flammable solids, category 2 Flammable liq. 2 Signal word :Danger Hazard statements: Highly flammable liquid and vapour Precautionary statements: If medical advice is needed, have product container or label at hand Keep out of reach of children Read label before use Keep away from heat/sparks/open flames/hot surfaces. No smoking Keep container tightly closed Ground/bond container and receiving equipment Use explosion-proof electrical/ventilating/light/…/equipment Use only non-sparking tools Take precautionary measures against static discharge Wear protective gloves/protective clothing/eye protection/face protection IF ON SKIN (or hair): Remove/Take off immediately all contaminated clothing. Rinse skin with water/shower In case of fire: Use … for extinction Store in a well ventilated place. Keep cool Dispose of contents/container to … Other Non-GHS Classification: WHMIS Created by Global Safety Management, Inc. -Tel: 1-813-435-5161 - www.gsmsds.com Safety Data Sheet according to 29CFR1910/1200 and GHS Rev. -

Chemical Chemical Hazard and Compatibility Information
Chemical Chemical Hazard and Compatibility Information Acetic Acid HAZARDS & STORAGE: Corrosive and combustible liquid. Serious health hazard. Reacts with oxidizing and alkali materials. Keep above freezing point (62 degrees F) to avoid rupture of carboys and glass containers.. INCOMPATIBILITIES: 2-amino-ethanol, Acetaldehyde, Acetic anhydride, Acids, Alcohol, Amines, 2-Amino-ethanol, Ammonia, Ammonium nitrate, 5-Azidotetrazole, Bases, Bromine pentafluoride, Caustics (strong), Chlorosulfonic acid, Chromic Acid, Chromium trioxide, Chlorine trifluoride, Ethylene imine, Ethylene glycol, Ethylene diamine, Hydrogen cyanide, Hydrogen peroxide, Hydrogen sulfide, Hydroxyl compounds, Ketones, Nitric Acid, Oleum, Oxidizers (strong), P(OCN)3, Perchloric acid, Permanganates, Peroxides, Phenols, Phosphorus isocyanate, Phosphorus trichloride, Potassium hydroxide, Potassium permanganate, Potassium-tert-butoxide, Sodium hydroxide, Sodium peroxide, Sulfuric acid, n-Xylene. Acetone HAZARDS & STORAGE: Store in a cool, dry, well ventilated place. INCOMPATIBILITIES: Acids, Bromine trifluoride, Bromine, Bromoform, Carbon, Chloroform, Chromium oxide, Chromium trioxide, Chromyl chloride, Dioxygen difluoride, Fluorine oxide, Hydrogen peroxide, 2-Methyl-1,2-butadiene, NaOBr, Nitric acid, Nitrosyl chloride, Nitrosyl perchlorate, Nitryl perchlorate, NOCl, Oxidizing materials, Permonosulfuric acid, Peroxomonosulfuric acid, Potassium-tert-butoxide, Sulfur dichloride, Sulfuric acid, thio-Diglycol, Thiotrithiazyl perchlorate, Trichloromelamine, 2,4,6-Trichloro-1,3,5-triazine -

Chemical Name Federal P Code CAS Registry Number Acutely
Acutely / Extremely Hazardous Waste List Federal P CAS Registry Acutely / Extremely Chemical Name Code Number Hazardous 4,7-Methano-1H-indene, 1,4,5,6,7,8,8-heptachloro-3a,4,7,7a-tetrahydro- P059 76-44-8 Acutely Hazardous 6,9-Methano-2,4,3-benzodioxathiepin, 6,7,8,9,10,10- hexachloro-1,5,5a,6,9,9a-hexahydro-, 3-oxide P050 115-29-7 Acutely Hazardous Methanimidamide, N,N-dimethyl-N'-[2-methyl-4-[[(methylamino)carbonyl]oxy]phenyl]- P197 17702-57-7 Acutely Hazardous 1-(o-Chlorophenyl)thiourea P026 5344-82-1 Acutely Hazardous 1-(o-Chlorophenyl)thiourea 5344-82-1 Extremely Hazardous 1,1,1-Trichloro-2, -bis(p-methoxyphenyl)ethane Extremely Hazardous 1,1a,2,2,3,3a,4,5,5,5a,5b,6-Dodecachlorooctahydro-1,3,4-metheno-1H-cyclobuta (cd) pentalene, Dechlorane Extremely Hazardous 1,1a,3,3a,4,5,5,5a,5b,6-Decachloro--octahydro-1,2,4-metheno-2H-cyclobuta (cd) pentalen-2- one, chlorecone Extremely Hazardous 1,1-Dimethylhydrazine 57-14-7 Extremely Hazardous 1,2,3,4,10,10-Hexachloro-6,7-epoxy-1,4,4,4a,5,6,7,8,8a-octahydro-1,4-endo-endo-5,8- dimethanonaph-thalene Extremely Hazardous 1,2,3-Propanetriol, trinitrate P081 55-63-0 Acutely Hazardous 1,2,3-Propanetriol, trinitrate 55-63-0 Extremely Hazardous 1,2,4,5,6,7,8,8-Octachloro-4,7-methano-3a,4,7,7a-tetra- hydro- indane Extremely Hazardous 1,2-Benzenediol, 4-[1-hydroxy-2-(methylamino)ethyl]- 51-43-4 Extremely Hazardous 1,2-Benzenediol, 4-[1-hydroxy-2-(methylamino)ethyl]-, P042 51-43-4 Acutely Hazardous 1,2-Dibromo-3-chloropropane 96-12-8 Extremely Hazardous 1,2-Propylenimine P067 75-55-8 Acutely Hazardous 1,2-Propylenimine 75-55-8 Extremely Hazardous 1,3,4,5,6,7,8,8-Octachloro-1,3,3a,4,7,7a-hexahydro-4,7-methanoisobenzofuran Extremely Hazardous 1,3-Dithiolane-2-carboxaldehyde, 2,4-dimethyl-, O- [(methylamino)-carbonyl]oxime 26419-73-8 Extremely Hazardous 1,3-Dithiolane-2-carboxaldehyde, 2,4-dimethyl-, O- [(methylamino)-carbonyl]oxime. -
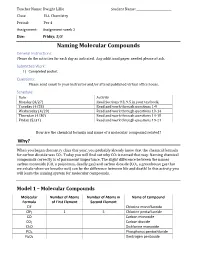
Naming Molecular Compounds General Instructions: Please Do the Activities for Each Day As Indicated
Teacher Name: Dwight Lillie Student Name: ________________________ Class: ELL Chemistry Period: Per 4 Assignment: Assignment week 2 Due: Friday, 5/8 Naming Molecular Compounds General Instructions: Please do the activities for each day as indicated. Any additional paper needed please attach. Submitted Work: 1) Completed packet. Questions: Please send email to your instructor and/or attend published virtual office hours. Schedule: Date Activity Monday (4/27) Read Sections 9.3, 9.5 in your textbook. Tuesday (4/28) Read and work through questions 1-9 Wednesday (4/29) Read and work through questions 10-14 Thursday (4/30) Read and work through questions 14-18 Friday (5/31) Read and work through questions 19-21 How are the chemical formula and name of a molecular compound related? Why? When you began chemistry class this year, you probably already knew that the chemical formula for carbon dioxide was CO2. Today you will find out why CO2 is named that way. Naming chemical compounds correctly is of paramount importance. The slight difference between the names carbon monoxide (CO, a poisonous, deadly gas) and carbon dioxide (CO2, a greenhouse gas that we exhale when we breathe out) can be the difference between life and death! In this activity you will learn the naming system for molecular compounds. Model 1 – Molecular Compounds Molecular Number of Atoms Number of Atoms in Name of Compound Formula of First Element Second Element ClF Chlorine monofluoride ClF5 1 5 Chlorine pentafluoride CO Carbon monoxide CO2 Carbon dioxide Cl2O Dichlorine monoxide PCl5 Phosphorus pentachloride N2O5 Dinitrogen pentoxide 1. Fill in the table to indicate the number of atoms of each type in the molecular formula. -

Website ( Or in Your Facility’S RTK Other Effects Central File Or Hazard Communication Standard File
Right to Know Hazardous Substance Fact Sheet Common Name: BROMINE PENTAFLUORIDE Synonyms: None CAS Number: 7789-30-2 Chemical Name: Bromine Fluoride RTK Substance Number: 0254 Date: July 1998 Revision: November 2007 DOT Number: UN 1745 Description and Use EMERGENCY RESPONDERS >>>> SEE BACK PAGE Bromine Pentafluoride is a colorless to pale yellow liquid with Hazard Summary o a strong odor. It becomes a gas at temperatures above 104 F Hazard Rating NJDOH NFPA o (40 C). It is used as an oxidizer and a fluorinating agent in HEALTH - 4 making Fluorocarbons. FLAMMABILITY - 0 REACTIVITY - 3 W WATER REACTIVE CORROSIVE STRONG OXIDIZER Reasons for Citation POISONOUS GASES ARE PRODUCED IN FIRE f Bromine Pentafluoride is on the Right to Know Hazardous CONTAINERS MAY EXPLODE IN FIRE Substance List because it is cited by ACGIH, DOT, NIOSH and NFPA. Hazard Rating Key: 0=minimal; 1=slight; 2=moderate; 3=serious; 4=severe f This chemical is on the Special Health Hazard Substance List. f Bromine Pentafluoride can affect you when inhaled. f Contact can severely irritate and burn the skin and eyes. f Inhaling Bromine Pentafluoride can irritate the nose and throat. SEE GLOSSARY ON PAGE 5. f Inhaling Bromine Pentafluoride can irritate the lungs. Higher exposures may cause a build-up of fluid in the lungs FIRST AID (pulmonary edema), a medical emergency. f Eye Contact Repeated exposure can cause headache, dizziness, nausea f Immediately flush with large amounts of water for at least 30 and vomiting. minutes, lifting upper and lower lids. Remove contact f Bromine Pentafluoride is not combustible but it is a lenses, if worn, while flushing. -

Interhalogen Compounds
INTERHALOGEN COMPOUNDS Smt. EDNA RICHARD Asst. Professor Department of Chemistry INTERHALOGEN COMPOUND An interhalogen compound is a molecule which contains two or more different halogen atoms (fluorine, chlorine, bromine, iodine, or astatine) and no atoms of elements from any other group. Most interhalogen compounds known are binary (composed of only two distinct elements) The common interhalogen compounds include Chlorine monofluoride, bromine trifluoride, iodine pentafluoride, iodine heptafluoride, etc Interhalogen compounds into four types, depending on the number of atoms in the particle. They are as follows: XY XY3 XY5 XY7 X is the bigger (or) less electronegative halogen. Y represents the smaller (or) more electronegative halogen. Properties of Interhalogen Compounds •We can find Interhalogen compounds in vapour, solid or fluid state. • A lot of these compounds are unstable solids or fluids at 298K. A few other compounds are gases as well. As an example, chlorine monofluoride is a gas. On the other hand, bromine trifluoride and iodine trifluoride are solid and liquid respectively. •These compounds are covalent in nature. •These interhalogen compounds are diamagnetic in nature. This is because they have bond pairs and lone pairs. •Interhalogen compounds are very reactive. One exception to this is fluorine. This is because the A-X bond in interhalogens is much weaker than the X-X bond in halogens, except for the F-F bond. •We can use the VSEPR theory to explain the unique structure of these interhalogens. In chlorine trifluoride, the central atom is that of chlorine. It has seven electrons in its outermost valence shell. Three of these electrons form three bond pairs with three fluorine molecules leaving four electrons. -

Halogen Fluorides Chlorine Trifluoride ( Clf3 )
Journal content Halogen fluorides Chlorine trifluoride ( ClF3), Bromine trifluoride (BrF3) Trifluorides of bromine and chlorine are strong fluorinating reagents and chlorine trifluoride is among the most reactive and aggressive compounds and does not yield to fluorine in chemical activity. Main constants of these compounds are as follows: ClF3 BrF3 Molecular mass 92.46 136.91 Boiling temperature,oC 11.75 125.75 Melting temperature, oC -76.3 8.77 Density at 25oC,g/cm3 1.8094 2.8 ClF3 was obtained and identified by O.Ruff and H.Krug in 1930 [2], BrF3 was produced by P.Leberau [3] and E.B.R.Prideaux [4] in 1905. Both halogen fluorides are produced in industry by direct fluorination of Cl2(Br2) in a nickel equipment followed by further purification from admixtures by fractional distillation [5]. Methods to produce ClF3 and BrF3, their chemical and physical properties, molecular structure etc. have been reviewed in detail in a number of papers including [1,6,7,8], while there are few data about their application except application in nuclear [1,8] and space [8] engineering. Since the reviews contain information up to the late 60-s ( 1966-1967), it seems expedient to examine improvements in a technology of production and purification of ClF3 and BrF3 and also data about their application published since 1967. 1. Technology of production and purification of ClF3 and BrF3 There are practically no records for the period under review (1967-1999). Three reports [9,10,11] relate to investigation of kinetics of reactions to produce ClF3 under specific conditions.