Chlorine Trifluoride Ctf
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

2018 Annual Survey of Biological and Chemical Agents Regulated by Homeland Security (And Carcinogens Regulated by OSHA)
Name: Dept: Date: 2018 Annual Survey of Biological and Chemical Agents regulated by Homeland Security (and carcinogens regulated by OSHA) Due (date) All labs that do not have a current chemical inventory in Chematix MUST complete this survey. The University is required to make an annual report of all chemicals on the Chemical Facility Anti-Terrorism Standards (CFATS) lists. Additional information regarding the regulations is available on the EH&S website at http://www.safety.rochester.edu/restricted/occsafe/chemicalagent.html and https://www.selectagents.gov. 1. Please review the lists on the following pages and indicate if any are possessed by your lab. The CAS# has been added to the list for ease of searching databases. The CAS# is a Chemical Abstract Service numbering system which assigns a unique number to every chemical substance based on structure; this helps avoid confusion by use of synonyms or different naming conventions. a. If yes for possession, place an X in the applicable box and if requested, include the quantity held in your lab. b. If no, leave blank. 2. After reviewing the list, please complete the information box below (or on last page for possession), then sign, date and return to EH&S. 3. Please call Donna Douglass at 275-2402 if you have any questions. Thank you for your cooperation in collecting data required by the Department of Homeland Security! Possession: 1) Fill in applicable boxes, 2) have PI sign last page, 3) return all pages to Donna Douglass OR Non-possession: 1) Check only one box on the left, 2) sign, 3) return just this page to Donna Douglass I do not have a lab, do not work in a lab, nor do I possess any of the agents in this survey. -

Aegls Brochure
4.85 5 5 About the Board on Environmental Studies and Toxicology The Board on Environmental Studies and Toxicology addresses Types of Chemicals Covered in the AEGLs Series environmental pollution problems affecting human health, human impacts on the environment, and the assessment and management of risks to AEGLs values for the chemicals listed below were published in the first human health and the environment. The board’s reports answer questions six volumes of the AEGLs series. AEGLs for additional chemicals will about air and water pollution; solid and hazardous waste; toxicology; continue to be published in subsequent volumes. epidemiology; risk assessment; applied ecology; natural resources; and environmental engineering, economics, law, and policy. Allylamine Hydrogen fluoride Ammonia Iron pentacarbonyl Aniline Methyl hydrazine Arsine Methyl isocyanate About NRC Reports from the National Academies Protecting Chlorine Nerve agents GA [tabun], The National Academies, through its National Research Council reports, Chlorine dioxide GB [sarin], GD [soman], GF, provides a unique public service by working outside the framework of Chlorine trifluoride and VX the Public and government to ensure independent, expert advice on matters of science, Crotonaldehyde Nickel carbonyl technology, and medicine. Today, the National Academies include three Cyclohexylamine Phosgene honorary societies that elect new members to their ranks each year- Diborane Phosphine Emergency the National Academy of Sciences, the National Academy of Engineering, 1,1-Dichloro-1-fluoroethane Propylene glycol dinitrate and the Institute of Medicine-and the National Research Council, the (HCFC-141B) Sulfur mustard operating arm that conducts the bulk of the institution’s Dimethylhydrazine 1,1,1,2-Tetrafluoroethane Workers science-policy and technical work. -

Chemical Chemical Hazard and Compatibility Information
Chemical Chemical Hazard and Compatibility Information Acetic Acid HAZARDS & STORAGE: Corrosive and combustible liquid. Serious health hazard. Reacts with oxidizing and alkali materials. Keep above freezing point (62 degrees F) to avoid rupture of carboys and glass containers.. INCOMPATIBILITIES: 2-amino-ethanol, Acetaldehyde, Acetic anhydride, Acids, Alcohol, Amines, 2-Amino-ethanol, Ammonia, Ammonium nitrate, 5-Azidotetrazole, Bases, Bromine pentafluoride, Caustics (strong), Chlorosulfonic acid, Chromic Acid, Chromium trioxide, Chlorine trifluoride, Ethylene imine, Ethylene glycol, Ethylene diamine, Hydrogen cyanide, Hydrogen peroxide, Hydrogen sulfide, Hydroxyl compounds, Ketones, Nitric Acid, Oleum, Oxidizers (strong), P(OCN)3, Perchloric acid, Permanganates, Peroxides, Phenols, Phosphorus isocyanate, Phosphorus trichloride, Potassium hydroxide, Potassium permanganate, Potassium-tert-butoxide, Sodium hydroxide, Sodium peroxide, Sulfuric acid, n-Xylene. Acetone HAZARDS & STORAGE: Store in a cool, dry, well ventilated place. INCOMPATIBILITIES: Acids, Bromine trifluoride, Bromine, Bromoform, Carbon, Chloroform, Chromium oxide, Chromium trioxide, Chromyl chloride, Dioxygen difluoride, Fluorine oxide, Hydrogen peroxide, 2-Methyl-1,2-butadiene, NaOBr, Nitric acid, Nitrosyl chloride, Nitrosyl perchlorate, Nitryl perchlorate, NOCl, Oxidizing materials, Permonosulfuric acid, Peroxomonosulfuric acid, Potassium-tert-butoxide, Sulfur dichloride, Sulfuric acid, thio-Diglycol, Thiotrithiazyl perchlorate, Trichloromelamine, 2,4,6-Trichloro-1,3,5-triazine -

Department of Homeland Security Chemicals of Interest
Department of Homeland Security Chemicals of Interest On October 4, 2006, President George W Bush signed the Homeland Security Appropriations Act of 2007. This Act gave the Department of Homeland Security (DHS) authority to regulate the security of facilities that contain high risk chemicals. On April 9, 2007, the Department of Homeland Security issued the Chemical Facility Anti- Terrorism Standards (CFATS). Under these standards the University must maintain an inventory of chemicals that are subject to this regulation. The chemicals subject to this regulation are more commonly known as chemicals of interest or COIs. In order to ensure the University of Northern Iowa remains compliant with this regulation, departments who come into possession of any of the chemicals listed on the following pages must immediately inform the Environmental Health and Safety (EH&S) Office of the name of the chemical or chemicals that were acquired, their respective amounts and storage location. Departments must also submit annual inventories of their chemicals to the EH&S office so an annual review of the amounts of COIs the University has on campus can be completed. If at any time it is found the amount of one or more of the COIs on campus exceeds the regulatory level (designated as threshold quantity by the DHS), the University must submit a report to the Department of Homeland Security (referred to as a “Top-Screen” by the DHS) within 60 days. Failure to report this information could result in fines to the University of $25,000 per day. The EH&S Office is responsible for gathering of campus chemical information and for submitting the Top Screen Report to the Department of Homeland Security on behalf of the University. -
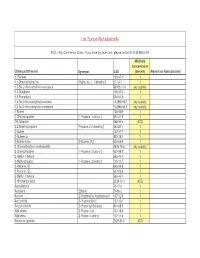
Homeland Security List
List Sorted Alphabetically ACG = Any Commercial Grade. If you have any questions, please contact EHS @ 898-5126. Minimum Concentration Chemical Of Interest Synonym CAS (percent) Amount on Hand (pounds) 1- Pentene 109-67-1 1 1,1-Dimethylhydrazine [Hydrazine, 1, 1-dimethyl-] 57-14-7 1 1,3-Bis(2-chloroethylthio)-n-propane 63905-10-2 any quantity 1,3-Butadiene 106-99-0 1 1,3-Pentadiene 504-60-9 1 1,4-Bis(2-chloroethylthio)-n-butane 142868-93-7 any quantity 1,5-Bis(2-chloroethylthio)-n-pentane 142868-94-8 any quantity 1-Butene 106-98-9 1 1-Chloropropylene [1-Propene, 1-chloro-] 590-21-6 1 1H-Tetrazole 288-94-8 ACG 2,2-Dimethylpropane [Propane, 2,2-dimethyl-] 463-82-1 1 2-Butene 107-01-7 1 2-Butene-cis 590-18-1 1 2-Butene-trans [2-Butene, (E)] 624-64-6 1 2-Chloroethylchloro-methylsulfide 2625-76-5 any quantity 2-Chloropropylene [1-Propene, 2-chloro-] 557-98-2 1 2-Methyl-1-butene 563-46-2 1 2-Methylpropene [1-Propene, 2-methyl-] 115-11-7 1 2-Pentene, (E)- 646-04-8 1 2-Pentene, (Z)- 627-20-3 1 3-Methyl-1-butene 563-45-1 1 5-Nitrobenzotriazol 2338-12-7 ACG Acetaldehyde 75-07-0 1 Acetylene [Ethyne] 74-86-2 1 Acrolein [2-Propenal] or Acrylaldehyde 107-02-8 1 Acrylonitrile [2-Propenenitrile] 107-13-1 1 Acrylyl chloride [2-Propenoyl chloride] 814-68-6 1 Allyl alcohol [2-Propen-1-ol] 107-18-6 1 Allylamine [2-Propen-1-amine] 107-11-9 1 Aluminum (powder) 7429-90-5 ACG Ammonia (anhydrous) 7664-41-7 1 Ammonia (conc. -

Chlorine Trifluoride
Safetygram #39 Chlorine Trifluoride Warning: Improper storage, handling or use of chlorine trifluoride can result in serious injury and/or prop- erty damage. Use this product in accordance with the Air Products Material Safety Data Sheet (MSDS). Introduction Chlorine trifluoride (ClF3) is a toxic, corrosive, very est in the use of ClF3 for organic synthesis work reactive liquefied compressed gas packaged in increased although the material was eventually cylinders as a liquid under its own vapor pressure considered to be too reactive for practical use 2 2 of 1.55 kg/cm at 21°C (22 psia at 70°F). ClF3 is and mostly abandoned for these applications. a very useful chemical in operations requiring a Synthesis reactions proved difficult to control and high-energy fluorinating agent or incendiary mate- usually led to a wide variety of reaction by- rial, especially since it can be handled at room products that were hazardous. temperatures. However, those same factors that make it quite useful also contribute to several high Many fluorinating compounds were evaluated as hazard potentials for the product. potent oxidizers for liquid-fueled rockets in the late 1940s through the early 1950s to overcome the Air Products has been the primary manufacturer storage and handling disadvantages of liquid F2. and distributor of ClF3 in North America for the past ClF3 was first tested in the U.S. in 1948 on a liquid 35 years, and the compound represents one of the propellant rocket motor using hydrazine as the highest-reactivity products that Air Products cur- fuel. Additional testing yielded favorable results. -

Emergency and Disaster Response to Chemical Releases
Emergency and Disaster Response to Chemical Releases Technician Level Training 29 CFR 1910.120(q) Module 3 Chemistry Awareness Emergency and Disaster Response to Chemical Releases This page left blank intentionally. ÓHMTRI Chemistry January 06 Page 32 Emergency and Disaster Response to Chemical Releases Table of Contents Acronyms Used in This Module................................................................5 Overview..................................................................................................7 Terminal Learning Objective.....................................................................7 Enabling Objectives..................................................................................7 Introduction ..............................................................................................9 Potential Hazardous Chemicals................................................................9 Physical and Chemical Properties............................................................9 Freezing Point.....................................................................................10 Boiling Point........................................................................................10 Melting Point .......................................................................................11 Vapor Pressure/Volatility.....................................................................11 Flammable Range, LEL, and UEL..........................................................12 Lower Explosive Limit (LEL)................................................................12 -

TIH/PIH List
Hazardous Materials Designated as TIH/PIH (consolidated AAR and Railinc lists) 3/12/2007 STCC Proper Shipping Name 4921402 2-CHLOROETHANAL 4921495 2-METHYL-2-HEPTANETHIOL 4921741 3,5-DICHLORO-2,4,6-TRIFLUOROPYRIDINE 4921401 ACETONE CYANOHYDRIN, STABILIZED 4927007 ACROLEIN, STABILIZED 4921019 ALLYL ALCOHOL 4923113 ALLYL CHLOROFORMATE 4921004 ALLYLAMINE 4904211 AMMONIA SOLUTION 4920360 AMMONIA SOLUTIONS 4904209 AMMONIA, ANHYDROUS 4904210 AMMONIA, ANHYDROUS 4904879 AMMONIA, ANHYDROUS 4920359 AMMONIA, ANHYDROUS 4923209 ARSENIC TRICHLORIDE 4920135 ARSINE 4932010 BORON TRIBROMIDE 4920349 BORON TRICHLORIDE 4920522 BORON TRIFLUORIDE 4936110 BROMINE 4920715 BROMINE CHLORIDE 4918505 BROMINE PENTAFLUORIDE 4936106 BROMINE SOLUTIONS 4918507 BROMINE TRIFLUORIDE 4921727 BROMOACETONE 4920343 CARBON MONOXIDE AND HYDROGEN MIXTURE, COMPRESSED 4920399 CARBON MONOXIDE, COMPRESSED 4920511 CARBON MONOXIDE, REFRIGERATED LIQUID 4920559 CARBONYL FLUORIDE 4920351 CARBONYL SULFIDE 4920523 CHLORINE 4920189 CHLORINE PENTAFLUORIDE 4920352 CHLORINE TRIFLUORIDE 4921558 CHLOROACETONE, STABILIZED 4921009 CHLOROACETONITRILE 4923117 CHLOROACETYL CHLORIDE 4921414 CHLOROPICRIN 4920516 CHLOROPICRIN AND METHYL BROMIDE MIXTURES 4920547 CHLOROPICRIN AND METHYL BROMIDE MIXTURES 4920392 CHLOROPICRIN AND METHYL CHLORIDE MIXTURES 4921746 CHLOROPIVALOYL CHLORIDE 4930204 CHLOROSULFONIC ACID 4920527 COAL GAS, COMPRESSED 4920102 COMMPRESSED GAS, TOXIC, FLAMMABLE, CORROSIVE, N.O.S. 4920303 COMMPRESSED GAS, TOXIC, FLAMMABLE, CORROSIVE, N.O.S. 4920304 COMMPRESSED GAS, TOXIC, FLAMMABLE, CORROSIVE, N.O.S. 4920305 COMMPRESSED GAS, TOXIC, FLAMMABLE, CORROSIVE, N.O.S. 4920101 COMPRESSED GAS, TOXIC, CORROSIVE, N.O.S. 4920300 COMPRESSED GAS, TOXIC, CORROSIVE, N.O.S. 4920301 COMPRESSED GAS, TOXIC, CORROSIVE, N.O.S. 4920324 COMPRESSED GAS, TOXIC, CORROSIVE, N.O.S. 4920331 COMPRESSED GAS, TOXIC, CORROSIVE, N.O.S. 4920165 COMPRESSED GAS, TOXIC, FLAMMABLE, N.O.S. 4920378 COMPRESSED GAS, TOXIC, FLAMMABLE, N.O.S. 4920379 COMPRESSED GAS, TOXIC, FLAMMABLE, N.O.S. -

Toxic and Highly Toxic Gas Handling Procedure
Environmental Health and Safety Toxic and Highly Toxic Gas Handling Procedure Date of Issuance: 10/2019 Revision Date: 5/27/2021 Revision Number: 1 Prepared by: EHS 1. Scope and Background The Carnegie Mellon University Toxic and Highly Toxic Gas Handling Program specifies minimum requirements for safe storage, use, and handling of toxic and highly toxic gas at Carnegie Mellon University. This program has been approved by the Laboratory Safety Committee. This document: • summarizes the health and safety risks associated with toxic and highly toxic gas use and handling; • identifies exposure control methods to protect employee’s safety and health and the environment; • outlines regulatory and university requirements related to this work; • specifies emergency response procedures for addressing toxic gas issues; and • provides resources for further information. Toxic and highly toxic gases are gases that may cause significant acute health effects at low concentrations. Health effects may include severe skin or eye irritation, pulmonary edema, neurotoxicity or other potentially fatal conditions. The criteria used to establish the materials addressed by this policy are: • A National Fire Protection Association (NFPA) health rating of 3 or 4 • An NFPA health rating of 2 with poor physiological warning properties • Pyrophoric (self-igniting) characteristics, OR • An extremely low occupational exposure limits in the absence of an NFPA health rating. Table 1 identifies common gases that meet the criteria of toxic or highly toxic, though it is -

Chemicals Requiring EHS Pre-Approval
Chemicals Requiring EHS Approval Before Purchasing Chemical Name CAS # 1,3-Bis(2-chloroethylthio)-n-propane 63905-10-2 1,4-Bis(2-chloroethylthio)-n-butane 142868-93-7 1,5-Bis(2-chloroethylthio)-n-pentane 142868-94-8 1H-Tetrazole 288-94-8 2-chloroethyl ethylsulfide 693-07-2 2-Chloroethylchloro-methylsulfide 2625-76-5 2-Cyanoethyl diisopropylchlorophosphoramidite 89992-70-1 2-Ethoxyethanol 110-80-5 2-Ethoxyethylacetate 111-15-9 2-Methoxyethanol 109-86-4 2-Methoxyethylacetate 110-49-6 5-Nitrobenzotriazol 2338-12-7 Acetone cyanohydrin, stabilized 75-86-5 Acrolein 107-02-8 Acrylamide (powder) 79-06-1 Allylamine 107-11-9 Aluminum (powder) 7429-90-5 Aluminum phosphide 20859-73-8 Ammonia 7664-41-7 Ammonium nitrate 6484-52-2 Ammonium perchlorate 7790-98-9 Ammonium picrate 131-74-8 Arsenic 7440-38-2 Arsenic trichloride 7784-34-1 Arsenic trioxide 1327-53-3 Arsine 7784-42-1 Barium azide 18810-58-7 Beryllium 7440-41-7 Bis(2-chloroethylthio)methane 63869-13-6 Bis(2-chloroethylthiomethyl)ether 63918-90-1 Boron tribromide 10294-33-4 Boron trichloride 10294-34-5 Boron trifluoride 7637-07-2 Bromine 7726-95-6 Bromine chloride 13863-41-7 Bromine pentafluoride 7789-30-2 Bromine trifluoride 7787-71-5 Cadmium 7440-43-9 Calcium phosphide 1305-99-3 Carbon monoxide 630-08-0 Carbonyl fluoride 353-50-4 Carbonyl sulfide 463-58-1 Chlorine 7782-50-5 Chlorine dioxide 10049-04-4 Chlorine pentafluoride 13637-63-3 Chlorine trifluoride 7790-91-2 Chemicals Requiring EHS Approval Before Purchasing Chloroacetyl chloride 79-04-9 Chlorosarin 1445-76-7 Chlorosoman 7040-57-5 Chlorosulfonic -

Guide for the Selection of Chemical Agent and Toxic Industrial Material Detection Equipment for Emergency First Responders
U.S. Department of Justice Office of Justice Programs National Institute of Justice National Institute of Justice Law Enforcement and Corrections Standards and Testing Program Guide for the Selection of Chemical Agent and Toxic Industrial Material Detection Equipment for Emergency First Responders NIJ Guide 100-00 Volume I June 2000 ABOUT THE LAW ENFORCEMENT AND CORRECTIONS STANDARDS AND TESTING PROGRAM The Law Enforcement and Corrections Standards and Testing Program is sponsored by the Office of Science and Technology of the National Institute of Justice (NIJ), U.S. Department of Justice. The program responds to the mandate of the Justice System Improvement Act of 1979, which directed NIJ to encourage research and development to improve the criminal justice system and to disseminate the results to Federal, State, and local agencies. The Law Enforcement and Corrections Standards and Testing Program is an applied research effort that determines the technological needs of justice system agencies, sets minimum performance standards for specific devices, tests commercially available equipment against those standards, and disseminates the standards and the test results to criminal justice agencies nationally and internationally. The program operates through: The Law Enforcement and Corrections Technology Advisory Council (LECTAC) consisting of nationally recognized criminal justice practitioners from Federal, State, and local agencies, which assesses technological needs and sets priorities for research programs and items to be evaluated and tested. The Office of Law Enforcement Standards (OLES) at the National Institute of Standards and Technology, which develops voluntary national performance standards for compliance testing to ensure that individual items of equipment are suitable for use by criminal justice agencies. -

Tychem® Responder® CSM Product Change Notice
DuPont Personal Protection 5401 Jefferson Davis Highway Richmond, VA 23234 November 9, 2011 To Whom It May Concern: This notice is effective for all Tychem® Responder® CSM garments manufactured on or after October 13, 2011 A component supplier for Tychem® Responder® CSM fabric has made a change to one of the film barrier layers used in this fabric. Because there is a change in permeation data for some chemicals, we are asking our customers who use Tychem® Responder® CSM garments to please review the data provided which compares permeation resistance to more than 400 chemicals of the original fabric and the new fabric. The user should use this revised information to determine if the new Tychem® Responder® CSM garments remain suitable for use for their specific application(s). Before using the Tychem® Responder® CSM garments in this box, please review the revised permeation data attached. This information is also available on http://safespec.dupont.com. It is the responsibility of the user to determine protection levels needed for their hazards and the suitability of Tychem® Responder® CSM garments for their use. DuPont apologizes for any inconvenience this change may have caused. If you have any questions about the published permeation data or about our Tychem® Responder® CSM offering in general, please call Customer Service at 1-800-931-3456. Safety is a core value at DuPont and we value the safety of our customers. Thank you for your business. Regards, Nick DelliCompagni North America Product Manager DuPont Protection Technologies Page