Chemicals Requiring EHS Pre-Approval
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Air Contaminants – Permissible Exposure Limits (Pels)
SUBPART Z -- TOXIC AND HAZARDOUS SUBSTANCES 1910.1000-AIR CONTAMINANTS An employee’s exposure to any substance listed in Table Z-1-A of this section shall be limited in accordance with the requirements of the following paragraphs of this section. (a) Table Z-1-A. Limits for Air Contaminants (1) & (2) Enforcement of Transitional Limits has expired. See Paragraph (3) for Limits. (3) Limits for Air Contaminants Columns. An employee’s exposure to any substance listed in Table Z-1-A shall not exceed the Time Weighted Average (TWA), Short Term Exposure Limit (STEL) and Ceiling Limit specified for that substance in Table Z-1-A. (4) Skin Designation. To prevent or reduce skin absorption, an employee’s skin exposure to substances listed in Table Z-1-A with an “X” in the Skin Designation column following the substance name shall be prevented or reduced to the extent necessary in the circumstances through the use of gloves, coveralls, goggles, or other appropriate personal protective equipment, engineering controls or work practices. (5) Definitions. The following definitions are applicable to the Limits for Air Contaminants columns of Table Z- 1-A: (i) Time weighted average (TWA) is the employee’s average airborne exposure in any 8-hour work shift of a 40-hour work week which shall not be exceeded. (ii) Short term exposure limit (STEL) is the employee’s 15-minute time weighted average exposure which shall not be exceeded at any time during a work day unless another time limit is specified in a parenthetical notation below the limit. -

Theoretical Study of Sarin Adsorption On
Chemical Physics Letters 738 (2020) 136816 Contents lists available at ScienceDirect Chemical Physics Letters journal homepage: www.elsevier.com/locate/cplett Research paper Theoretical study of sarin adsorption on (12,0) boron nitride nanotube doped with silicon atoms T ⁎ ⁎ Jeziel Rodrigues dos Santosa, , Elson Longo da Silvab, Osmair Vital de Oliveirac, , José Divino dos Santosa a Universidade Estadual de Goiás, Campus Anápolis, CEP: 75.132-903 GO, Brazil b INCTMN, LIEC, Departamento de Química da Universidade Federal de São Carlos, CEP: 13.565-905 São Carlos, SP, Brazil c Instituto Federal de Educação, Ciência e Tecnologia de São Paulo, Campus Catanduva, CEP: 15.808-305 Catanduva, SP, Brazil HIGHLIGHTS • DFT method was used to study the adsorption of nerve agent sarin by BNNT. • Electronic properties of pristine BNNT are improved by Si impurity atoms. • The adsorption of sarin by Si-doped BNNT is highest favorable than the pure BNNT. • Si-doped BNNT can be a new gas sensor for sarin gas detection and its derivatives. ARTICLE INFO ABSTRACT Keywords: Sarin gas is one of the most lethal nerve agent used in chemical warfare, which its detection is import to prevent Nerve agent sarin a chemical attack and to identify a contamination area. Herein, density functional theory was used to investigate Gas sensor the (12,0) boron nitride nanotube (BNNT) and Si–doped BNNT as possible candidates to sarin detection. The Si- Boron nitride nanotube atoms doped improve the electronic properties of nanotubes by altering the electrostatic potential, HOMO and DFT LUMO energies. Based in the adsorption energies and the conductivity increased to ~33 and 350%, respectively, for Si- and 2Si-BNNT imply that they can be used for sarin detection. -

Industry Compliance Programme
Global Chemical Industry Compliance Programme GC-ICP Chemical Weapons Convention December 2006 Version 1.0 GLOBAL CHEMICAL INDUSTRY COMPLIANCE PROGRAMME FOR IMPLEMENTING THE CHEMICAL WEAPONS CONVENTION The purpose of the handbook is to provide guidance to chemical facilities, traders and trading companies in developing a Global Chemical Industry Compliance Programme (GC-ICP) to comply with the Chemical Weapons Convention (CWC). The GC-ICP focuses first on determining if there is a reporting requirement to your National Authority and second on collecting the relevant support data used to complete the required reports. The GC-ICP is designed to provide a methodology to comply with the CWC and establish systems that facilitate and demonstrate such compliance. Each facility/company should also ensure that it follows its country’s CWC specific laws, regulations and reporting requirements. • Sections 2, 3, and 4 guide you through the process of determining if chemicals at your facility/ company should be reported to your National Authority for compliance with the CWC. • Section 5 provides recommended guidance on information that you may use to determine your reporting requirements under the CWC and administrative tools that your facility/company may use to ensure compliance with the CWC. • Section 6 provides a glossary of terms and associated acronyms. • Section 7 provides a listing of all National Authorities by country. CWC Global Chemical Industry Compliance Programme 1 TABLE OF CONTENTS Section 1 Overview What is the Chemical Weapons Convention? -

Transport of Dangerous Goods
ST/SG/AC.10/1/Rev.16 (Vol.I) Recommendations on the TRANSPORT OF DANGEROUS GOODS Model Regulations Volume I Sixteenth revised edition UNITED NATIONS New York and Geneva, 2009 NOTE The designations employed and the presentation of the material in this publication do not imply the expression of any opinion whatsoever on the part of the Secretariat of the United Nations concerning the legal status of any country, territory, city or area, or of its authorities, or concerning the delimitation of its frontiers or boundaries. ST/SG/AC.10/1/Rev.16 (Vol.I) Copyright © United Nations, 2009 All rights reserved. No part of this publication may, for sales purposes, be reproduced, stored in a retrieval system or transmitted in any form or by any means, electronic, electrostatic, magnetic tape, mechanical, photocopying or otherwise, without prior permission in writing from the United Nations. UNITED NATIONS Sales No. E.09.VIII.2 ISBN 978-92-1-139136-7 (complete set of two volumes) ISSN 1014-5753 Volumes I and II not to be sold separately FOREWORD The Recommendations on the Transport of Dangerous Goods are addressed to governments and to the international organizations concerned with safety in the transport of dangerous goods. The first version, prepared by the United Nations Economic and Social Council's Committee of Experts on the Transport of Dangerous Goods, was published in 1956 (ST/ECA/43-E/CN.2/170). In response to developments in technology and the changing needs of users, they have been regularly amended and updated at succeeding sessions of the Committee of Experts pursuant to Resolution 645 G (XXIII) of 26 April 1957 of the Economic and Social Council and subsequent resolutions. -

Rhode Island Hazardous Substance List
Rhode Island Hazardous Substance List Source: T - ACGIH F - NFPA49 C - IARC Alphabetical Order C.A.S. ACGIH NFPA IARC CHEMICAL NAME 13010-47-4 C 1,-(2-Chloroethyl)-3-cyclohexyl-1-Nitrosourea 76-11-9 T 1,1,1,2-tetrachloro-2,2-difluoroethane 76-12-0 T 1,1,2,2-tetrachloro-1,2-difluoroethane 79-34-5 T 1,1,2,2-tetrachloroethane - skin 76-13-1 T 1,1,2-trichloro-1,2,2-trifluoroethane 79-00-5 T F C 1,1,2-trichloroethane - skin 594-72-9 T 1,1-Dichloro-1-nitroethane 74-34-3 T 1,1-dichloroethane 57-14-7 T 1,1-dimethylhydrazine (udmh) 96-18-4 T 1,2,3-trichloropropane 120-82-1 T 1,2,4-Trichlorobenzene 106-88-7 F 1,2-Butylene oxide 107-15-3 T F 1,2-Diaminoethane 96-12-8 C 1,2-Dibromo-3-chloropropane 106-93-4 T F C 1,2-Dibromoethane - skin 107-06-2 T F 1,2-Dichlorethane 540-59-0 T F 1,2-Dichloroethene 540-59-0 T F 1,2-Dichloroetylene 1615-80-1 C 1,2-Diethylhydrazine C 1,2-Dimethyl hydrazine - skin 106-99-0 T F 1,3-Butadiene 118-52-5 T 1,3-Dichloro-5,5-dimethylhydantoin 542-75-6 T F 1,3-Dichloropropene (cis and trans) 542-75-6 T F 1,3-Dichloropropylene 110-56-5 F 1,4-Dichlorobutane 123-91-1 T F C 1,4-Dioxane 1120-71-4 1-3-Propane sultone 110-53-2 F 1-Bromopentane 106-89-8 T F C 1-Chloro,2,3-epoxy-propane 600-25-9 T 1-Chloro-1-nitropropane 97-00-7 F 1-chloro-2,4-dinitrobenzene 543-59-9 F 1-Chloropentane 112-30-1 F 1-Decanol 111-27-3 F 1-Hexanol 141-79-7 T F 1-Isobutenyl methyl ketone 108-03-2 T F 1-Nitropropane 71-41-0 F 1-Pentanol 110-58-7 F 1-Pentylamine 111-40-0 T F 2,2'-Diaminodiethylamine 111-44-4 F 2,2'Dichlorodiethyl ether 75-99-0 T 2,2-dichloropropionic acid 556-52-5 T 2,3-Epoxy-1-propanol 93-76-5 T 2,4,5-T 95-95-4 F 2,4,5-trichlorophenol 88-06-2 F C 2,4,6-trichlorophenol 118-96-7 T F 2,4,6-Trinitro Toluene 479-95-8 T 2,4,6-Trinitrophenyl-methylnitramine 94-75-7 T 2,4-d (2,4-dichlorophenoxyacetic acid) 97-02-9 F 2,4-dinitroaniline 584-84-9 T F 2,4-Tolylene diisocyanate 108-83-8 T 2,6-Dimethyl-4-heptanone 108-83-8 T 2,6-Dimethyl-4-heptanone 128-37-0 T 2,6-Ditert. -

A Quantum Chemical Study Involving Nitrogen Mustards
The Pharmaceutical and Chemical Journal, 2016, 3(4):58-60 Available online www.tpcj.org ISSN: 2349-7092 Research Article CODEN(USA): PCJHBA Formation enthalpy and number of conformers as suitable QSAR descriptors: a quantum chemical study involving nitrogen mustards Robson Fernandes de Farias Universidade Federal do Rio Grande do Norte, Cx. Postal 1664, 59078-970, Natal-RN, Brasil Abstract In the present work, a quantum chemical study (Semi-empirical,PM6 method) is performed using nitrogen mustards (HN1, HN2 and HN3) as subjects in order to demonstrate that there is a close relationship between pharmacological activity and parameters such as formation enthalpy and number of conformers, which could, consequently, be employed as reliable QSAR descriptors. To the studied nitrogen mustards, a very simple equation o o relating log P, ΔH f and the number of conformers (Nc) was found: log P = [(log -ΔH f + logNc)/2]-0.28. Keywords QSAR, Descriptors, Formation enthalpy, Conformers, Semi-empirical, Nitrogen mustards, Log P Introduction It is well known that lipophilicity is a very important molecular descriptor that often correlates well with the bioactivity of chemicals [1]. Hence, lipophilicity, measured as log P, is a key property in quantitative structure activity relationship (QSAR) studies. In this connection, in the pharmaceutical sciences it is a common practice to use log P (the partition coefficient between water and octanol), as a reliable indicator of the hydrophobicity or lipophilicity of (drug) molecules [1-2]. For example, relying primarily on the log P is a sensible strategy in preparing future 18-crown-6 analogs with optimized biological activity [3]. -

The Chemotherapy of Malignant Disease -Practical and Experimental Considerations
Postgrad Med J: first published as 10.1136/pgmj.41.475.268 on 1 May 1965. Downloaded from POSTGRAD. MED. J. (1965), 41,268 THE CHEMOTHERAPY OF MALIGNANT DISEASE -PRACTICAL AND EXPERIMENTAL CONSIDERATIONS JOHN MATTHIAS, M.D., M.R.C.P., F.F.A., R.C.S. Physician, The Royal Marsden Hospital, London, S.W.3. THE TERM chemotherapy was introduced by positively charged alkyl (CH2) radicles of Ehrlich to describe the specific and effective the agent. treatment of infectious disease by chemical (a) The nitrogen mustards: mustine (HN2 substances. It is currently also applied to the 'nitrogen mustard', mechlorethamine, treatment of malignant disease. Unfortunately mustargen), trimustine (Trillekamin no aspect of tumour metabolism has been HN3), chlorambucil (Leukeran, phenyl discovered which has allowed the development butyric mustard), melphalan (Alkeran, of drugs capable of acting specifically upon the phenyl alanine mustard), uramustine malignant cell, so that cytotoxic drugs also (Uracil mustard), cyclophosphamide affect normal cells to a greater or lesser degree. (Endoxan or Cytoxan), mannomustine The most susceptible or sensitive of the normal (DegranoO). tissues are those with the highest rates of cell (b) The ethylenamines: tretamine (trie- turnover and include the haemopoietic and thanomelamine, triethylene melamine, lympho-reticular tissues, the gastro-intestinal TEM), thiotepa (triethylene thiopho- the the testis and the hair epithelium, ovary, sphoramide), triaziquone (Trenimon).by copyright. follicles. (c) The epoxides: triethyleneglycoldigly- Cancer chemotherapy may be said to encom- cidyl ether (Epodyl). pass all treatments of a chemical nature (d) The sulphonic acid esters: busulphan administered to patients with the purpose of (Myleran), mannitol myleran. restricting tumour growth or destroying tumour 2. -

Copyrighted Material
1 Historical Milieu 1.1 Organophosphorus Nerve Agents 2 1.2 Blister Agents 5 1.3 Sternutator Agents 11 1.4 Chemical Weapons Convention (CWC) 13 1.4.1 Schedule of Chemicals 14 1.4.2 Destruction of Chemical Weapons 14 References 16 COPYRIGHTED MATERIAL Analysis of Chemical Warfare Degradation Products, First Edition. Karolin K. Kroening, Renee N. Easter, Douglas D. Richardson, Stuart A. Willison and Joseph A. Caruso. © 2011 John Wiley & Sons, Ltd. Published 2011 by John Wiley & Sons, Ltd. 2 ANALYSIS OF CHEMICAL WARFARE DEGRADATION PRODUCTS 1.1 ORGANOPHOSPHORUS NERVE AGENTS Organophosphorus (OP) type compounds, that is, deriva- tives containing the P=O moiety, were first discovered in the 1800s when researchers were investigating useful applica- tions for insecticides/rodenticides. There are many derivatives of organophosphorus compounds, however, the OP deriva- tives that are typically known as ‘nerve agents’ were discov- ered accidentally in Germany in 1936 by a research team led by Dr. Gerhard Schrader at IG Farben [1–4]. Schrader had noticed the effects and lethality of these organophosphorus compounds towards insects and began developing a new class of insecticides. While working towards the goal of an improved insecticide, Schrader experimented with numerous phosphorus-containing compounds, leading to the discovery of the first nerve agent, Tabun (or GA) (Figure 1.1). The potency of these insecticides towards humans was not realized until there was yet another accident, which involved a Tabun spill. Schrader and coworkers began experiencing symptoms, such as miosis (constriction of the pupils of the eyes), dizziness and severe shortness of breath, with numerous effects lasting several weeks [1, 4, 5]. -

2018 Annual Survey of Biological and Chemical Agents Regulated by Homeland Security (And Carcinogens Regulated by OSHA)
Name: Dept: Date: 2018 Annual Survey of Biological and Chemical Agents regulated by Homeland Security (and carcinogens regulated by OSHA) Due (date) All labs that do not have a current chemical inventory in Chematix MUST complete this survey. The University is required to make an annual report of all chemicals on the Chemical Facility Anti-Terrorism Standards (CFATS) lists. Additional information regarding the regulations is available on the EH&S website at http://www.safety.rochester.edu/restricted/occsafe/chemicalagent.html and https://www.selectagents.gov. 1. Please review the lists on the following pages and indicate if any are possessed by your lab. The CAS# has been added to the list for ease of searching databases. The CAS# is a Chemical Abstract Service numbering system which assigns a unique number to every chemical substance based on structure; this helps avoid confusion by use of synonyms or different naming conventions. a. If yes for possession, place an X in the applicable box and if requested, include the quantity held in your lab. b. If no, leave blank. 2. After reviewing the list, please complete the information box below (or on last page for possession), then sign, date and return to EH&S. 3. Please call Donna Douglass at 275-2402 if you have any questions. Thank you for your cooperation in collecting data required by the Department of Homeland Security! Possession: 1) Fill in applicable boxes, 2) have PI sign last page, 3) return all pages to Donna Douglass OR Non-possession: 1) Check only one box on the left, 2) sign, 3) return just this page to Donna Douglass I do not have a lab, do not work in a lab, nor do I possess any of the agents in this survey. -

Information Data Sheet Detector Tube QUALITEST QL Part No. (US): 497665 Part No. D5085810
Information Data Sheet Detector Tube QUALITEST QL Part No. (US): 497665 Part No. D5085810 1. Application Detction of dangerous (toxic or combustible) gases and vapors in air.In particular fot testing the air in confined spaces such as fuel tanks, storage bins, cable vaults, sewers. Furthermore the tube QL can be used to detect or localize leaks, e.g. in pipelines. 2. General Description The detector tube QL does not have any calibration scale. The indication is non-specific and qualita- tive, i. e. only the presence resp. the absence of contaminants will be detected. The indication gives no information about type or concentration of contaminants detected. A special detector tube with quantitative indication shoud be used if the presence or the concentration of a known contaminant is to be determined. This applies also to substances which can not be indicated by the detector tube QL. The various contaminants are indicated with different sensitivity. However, the detector tube is sufficiently sensitive for all listed substances. Normally, concentrations of a few ppm are detectable. Among others, the following substances are indicated: Acetone, acetylene, benzene, 1.3-butadiene, butanes, butylenes, carbon disulfide, carbon monoxide, cyclohexane, diesel oil, ethanol (ethyl alcohol), ethylene, formic acid, fuel oil, gasoline (engine fuel),hydrogen chloride, hydrogen sulfide, kerosene, liquid petroleum gas (propane, butanes), methyl ethyl ketone (butanone), pentanes and other saturated hydrocarbons, phenol, propane, propanols (propyl alcohols), -
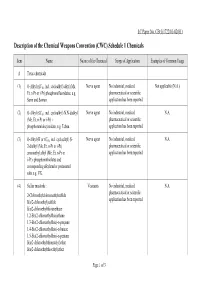
Description of the Chemical Weapons Convention (CWC) Schedule 1 Chemicals
LC Paper No. CB(1)1722/01-02(01) Description of the Chemical Weapons Convention (CWC) Schedule 1 Chemicals Item Name Nature of the Chemical Scope of Application Examples of Common Usage A Toxic chemicals (1) O-Alkyl (≤C10, incl. cycloalkyl) alkyl (Me, Nerve agent No industrial, medical, Not applicable (N.A.) Et, n-Pr or i-Pr) phosphonofluoridates, e.g. pharmaceutical or scientific Sarin and Soman. application has been reported. (2) O-Alkyl (≤C10, incl. cycloalkyl) N,N-dialkyl Nerve agent No industrial, medical, N.A. (Me, Et, n-Pr or i-Pr) - pharmaceutical or scientific phosphoramidocyanidate, e.g. Tabun. application has been reported. (3) O-Alkyl (H or ≤C10, incl. cycloalkyl) S- Nerve agent No industrial, medical, N.A. 2-dialkyl (Me, Et, n-Pr or i-Pr) pharmaceutical or scientific aminoethyl alkyl (Me, Et, n-Pr or application has been reported. i-Pr)- phosphonothiolates and corresponding alkylated or protonated salts e.g. VX. (4) Sulfur mustards : Vesicants No industrial, medical, N.A. pharmaceutical or scientific 2-Chloroethylchloromethylsulfide application has been reported. Bis(2-chloroethyl)sulfide Bis(2-chloroethylthio)methane 1,2-Bis(2-chloroethylthio)ethane 1,3-Bis(2-chloroethylthio)-n-propane 1,4-Bis(2-chloroethylthio)-n-butane 1,5-Bis(2-chloroethylthio)-n-pentane Bis(2-chloroethylthiomethyl)ether Bis(2-chloroethylthioethyl)ether Page 1 of 3 Item Name Nature of the Chemical Scope of Application Examples of Common Usage (5) Lewisites : Vesicants No industrial, medical, N.A. pharmaceutical or scientific Lewisite 1 : 2-Chlorovinyldichloroarsine application has been reported. Lewisite 2 : Bis(2-chlorovinyl)chloroarsine Lewisite 3 : Tris(2-chlorovinyl)arsine (6) Nitrogen mustards : Vesicants The chemical has medical Only HN2 has been reported to application. -

Anaerobic Degradation of Methanethiol in a Process for Liquefied Petroleum Gas (LPG) Biodesulfurization
Anaerobic degradation of methanethiol in a process for Liquefied Petroleum Gas (LPG) biodesulfurization Promotoren Prof. dr. ir. A.J.H. Janssen Hoogleraar in de Biologische Gas- en waterreiniging Prof. dr. ir. A.J.M. Stams Persoonlijk hoogleraar bij het laboratorium voor Microbiologie Copromotor Prof. dr. ir. P.N.L. Lens Hoogleraar in de Milieubiotechnologie UNESCO-IHE, Delft Samenstelling promotiecommissie Prof. dr. ir. R.H. Wijffels Wageningen Universiteit, Nederland Dr. ir. G. Muyzer TU Delft, Nederland Dr. H.J.M. op den Camp Radboud Universiteit, Nijmegen, Nederland Prof. dr. ir. H. van Langenhove Universiteit Gent, België Dit onderzoek is uitgevoerd binnen de onderzoeksschool SENSE (Socio-Economic and Natural Sciences of the Environment) Anaerobic degradation of methanethiol in a process for Liquefied Petroleum Gas (LPG) biodesulfurization R.C. van Leerdam Proefschrift ter verkrijging van de graad van doctor op gezag van de rector magnificus van Wageningen Universiteit Prof. dr. M.J. Kropff in het openbaar te verdedigen op maandag 19 november 2007 des namiddags te vier uur in de Aula Van Leerdam, R.C., 2007. Anaerobic degradation of methanethiol in a process for Liquefied Petroleum Gas (LPG) biodesulfurization. PhD-thesis Wageningen University, Wageningen, The Netherlands – with references – with summaries in English and Dutch ISBN: 978-90-8504-787-2 Abstract Due to increasingly stringent environmental legislation car fuels have to be desulfurized to levels below 10 ppm in order to minimize negative effects on the environment as sulfur-containing emissions contribute to acid deposition (‘acid rain’) and to reduce the amount of particulates formed during the burning of the fuel. Moreover, low sulfur specifications are also needed to lengthen the lifetime of car exhaust catalysts.