Industry Compliance Programme
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Responding to a Chemical Warfare Agent Incident: from Sampling and Analysis to Decontamination and Waste Management Stuart Willi
Responding to a Chemical Warfare Agent Incident: from sampling and analysis to decontamination and waste management Stuart Willison & Lukas Oudejans U. S. EPA National Homeland Security Research Center 1 Outline • Homeland Security Relevance to Chemical (Warfare Agent) Incidents and Incident Response Cycle • Identification of Gaps/Needs: PARTNER Process and Stakeholder Priorities • Current High Stakeholder Priorities • Research Efforts to meet these Needs/Gaps Selected Analytical Methods (SAM) Document CWA Method Development and Wipe Efficiency Studies on Surfaces Fate and Transport of CWAs Natural Attenuation of VX Decontamination of Vesicant/Blister CWAs HD, L, HL Analytical Method Development: Lewisite; EA 2192 Best Practices Document for Waste Media from Remediation Activities • Summary 2 Response to Contamination Events Since 9/11, multiple chemical/biotoxin contamination events have occurred in the United States and worldwide: • Several ricin incidents (2002-2014) • Deepwater Horizon oil spill (April 2010) • Kalamazoo River oil spill (July 2010) • CWA sulfur mustard clam shells (2010) • CWA chemical attacks (Syria, Middle East) (March-August 2013 and April 2014-current) • Elk River chemical spill in West Virginia (January 2014) • Toxic algae blooms in Toledo, OH (August 2014) • Arsenic-contaminated soil in Kentucky potentially containing CWA Lewisite (March 2015) • (Organophosphate-) Pesticide over- or misuse across USA in relation to bed bug epidemic (current) 3 Response Cycle Contaminant Release Reduce Vulnerabilities Lessons -

A Quantum Chemical Study Involving Nitrogen Mustards
The Pharmaceutical and Chemical Journal, 2016, 3(4):58-60 Available online www.tpcj.org ISSN: 2349-7092 Research Article CODEN(USA): PCJHBA Formation enthalpy and number of conformers as suitable QSAR descriptors: a quantum chemical study involving nitrogen mustards Robson Fernandes de Farias Universidade Federal do Rio Grande do Norte, Cx. Postal 1664, 59078-970, Natal-RN, Brasil Abstract In the present work, a quantum chemical study (Semi-empirical,PM6 method) is performed using nitrogen mustards (HN1, HN2 and HN3) as subjects in order to demonstrate that there is a close relationship between pharmacological activity and parameters such as formation enthalpy and number of conformers, which could, consequently, be employed as reliable QSAR descriptors. To the studied nitrogen mustards, a very simple equation o o relating log P, ΔH f and the number of conformers (Nc) was found: log P = [(log -ΔH f + logNc)/2]-0.28. Keywords QSAR, Descriptors, Formation enthalpy, Conformers, Semi-empirical, Nitrogen mustards, Log P Introduction It is well known that lipophilicity is a very important molecular descriptor that often correlates well with the bioactivity of chemicals [1]. Hence, lipophilicity, measured as log P, is a key property in quantitative structure activity relationship (QSAR) studies. In this connection, in the pharmaceutical sciences it is a common practice to use log P (the partition coefficient between water and octanol), as a reliable indicator of the hydrophobicity or lipophilicity of (drug) molecules [1-2]. For example, relying primarily on the log P is a sensible strategy in preparing future 18-crown-6 analogs with optimized biological activity [3]. -

Copyrighted Material
1 Historical Milieu 1.1 Organophosphorus Nerve Agents 2 1.2 Blister Agents 5 1.3 Sternutator Agents 11 1.4 Chemical Weapons Convention (CWC) 13 1.4.1 Schedule of Chemicals 14 1.4.2 Destruction of Chemical Weapons 14 References 16 COPYRIGHTED MATERIAL Analysis of Chemical Warfare Degradation Products, First Edition. Karolin K. Kroening, Renee N. Easter, Douglas D. Richardson, Stuart A. Willison and Joseph A. Caruso. © 2011 John Wiley & Sons, Ltd. Published 2011 by John Wiley & Sons, Ltd. 2 ANALYSIS OF CHEMICAL WARFARE DEGRADATION PRODUCTS 1.1 ORGANOPHOSPHORUS NERVE AGENTS Organophosphorus (OP) type compounds, that is, deriva- tives containing the P=O moiety, were first discovered in the 1800s when researchers were investigating useful applica- tions for insecticides/rodenticides. There are many derivatives of organophosphorus compounds, however, the OP deriva- tives that are typically known as ‘nerve agents’ were discov- ered accidentally in Germany in 1936 by a research team led by Dr. Gerhard Schrader at IG Farben [1–4]. Schrader had noticed the effects and lethality of these organophosphorus compounds towards insects and began developing a new class of insecticides. While working towards the goal of an improved insecticide, Schrader experimented with numerous phosphorus-containing compounds, leading to the discovery of the first nerve agent, Tabun (or GA) (Figure 1.1). The potency of these insecticides towards humans was not realized until there was yet another accident, which involved a Tabun spill. Schrader and coworkers began experiencing symptoms, such as miosis (constriction of the pupils of the eyes), dizziness and severe shortness of breath, with numerous effects lasting several weeks [1, 4, 5]. -

US5308512.Pdf
|||||||||||||| USOO530852A United States Patent (19) 11 Patent Number: 5,308,512 Stoll et al. 45 Date of Patent: May 3, 1994 54 THIODIGLYCOLALKOXYLATE 4010606 10/1991 Fed. Rep. of Germany. DERIVATIVES, A PROCESS FOR THEIR 0213067 12/1983 Japan. PRODUCTION AND THEIR USE AS FABRIC 153309 3/1984 Japan. SOFTENERS 1097396 1/1968 United Kingdom . (75) Inventors: Gerhard Stoll, Korschenbroich; OTHER PUBLICATIONS Peter Daute, Essen; Ingo Wegener; Trofimov, Zh. Prikl. Khim., vol. 48, pp. 626-628 1975. Faize Berger, both of Duesseldorf, all Vogel & Krissman & Seifen, Öle, Fette, Wachse, vol. of Fed. Rep. of Germany 115, 1989, pp. 3-8 Article Unavailable. (73) Assignee: Henkel Kommanditgesellschaft auf J. Falbe, Surfactants in Consumer Products, Springer, Aktien, Duesseldorf, Fed. Rep. of 1987, pp. 87-90. Germany Vogel & Krussmann, Seifen, Öle, Fette, Wachse, vol. (21) Appl. No.: 961,683 III, 1985, pp. 567-574 1985. Primary Examiner-Paul Lieberman (22) PCT Filed: Jun. 28, 1991 Assistant Examiner-Michael P. Tierney 86) PCT No.: PCT/EP91/01213 Attorney, Agent, or Firm-Ernest G. Szoke; Wayne C. S371 Date: Mar. 5, 1993 Jaeschke; Real J. Grandmaison S 102(e) Date: Mar. 5, 1993 57) ABSTRACT The process of producing alkoxylated thiodiglycol sulf 87). PCT Pub. No.: WO92/00959 oxide derivatives and thiodiglycol sulfone derivatives PCT Pub. Date: Jan. 23, 1992 corresponding to formula I (30) Foreign Application Priority Data Jul. 7, 1990 (DE) Fed. Rep. of Germany ....... 402,694 CH-CH-O-CH-CHR)-o-x, (I) (O)S 51) Int. Cl. ............................................ D06M 10/08 N 52 U.S.C. ...................................... 252/8.7; 252/8.6; CH-CH2-(O-CH-CHR)-O-X 252/8.9; 568/27; 568/28; 554/227; 560/263; 560/264 wherein X1 and X2 may be the same or different and 58 Field of Search ............... -

Chapter III. a Review of Spiking Chemicals Used in the First 40 OPCW Proficiency Tests
RECOMMENDED OPERATION PROCEDURES FOR CWC-RELATED ANALYSIS Section 5. Reporting Chapter III. A review of spiking chemicals used in the first 40 OPCW Proficiency Tests Chapter III. A review of spiking chemicals used in the first 40 OPCW Proficiency Tests Authors Keith Norman, Stephen Johnson Cranfield Forensic Institute Cranfield University Defence Academy of the United Kingdom Shrivenham, Swindon SN6 8LA, UK E-mail: [email protected], [email protected] Reviewers Hugh Gregg Organisation for the Prohibition of Chemical Weapons Johan de Wittlaan 32, 2517 JR, The Hague, The Netherlands E-mail: [email protected] Harri Kiljunen Finnish Institute for Verification of the Chemical Weapons Convention (VERIFIN) P.O. Box 55, FI-00014 University of Helsinki, Finland E-mail: [email protected] Chua Hoe Chee DSO National Laboratories, 12 Science Park Drive, Singapore 118225 E-mail: [email protected] Peter Siegenthaler Spiez Laboratory, Austrasse,CH-3700 Spiez, Switzerland E-mail: [email protected] 1. Scope From 1997 until 2016, the Organisation for the Prohibition of Chemical Weapons (OPCW) has coordinated 40 proficiency tests for the analysis and identification of intact chemical warfare agents, precursor chemicals, degradation and reaction products. This chapter reviews the chemicals used to spike the proficiency test samples, identifying those that have been used multiple times and the distribution of chemicals based upon the schedules in the chemical warfare convention (CWC). The aim of this chapter is not to provide an easy route to pass the proficiency tests but rather to illustrate the range of chemicals that should be considered during method development and/or validation for laboratories participating in, or considering participating in the OPCW Proficiency Test regime. -

Nerve Agent - Lntellipedia Page 1 Of9 Doc ID : 6637155 (U) Nerve Agent
This document is made available through the declassification efforts and research of John Greenewald, Jr., creator of: The Black Vault The Black Vault is the largest online Freedom of Information Act (FOIA) document clearinghouse in the world. The research efforts here are responsible for the declassification of MILLIONS of pages released by the U.S. Government & Military. Discover the Truth at: http://www.theblackvault.com Nerve Agent - lntellipedia Page 1 of9 Doc ID : 6637155 (U) Nerve Agent UNCLASSIFIED From lntellipedia Nerve Agents (also known as nerve gases, though these chemicals are liquid at room temperature) are a class of phosphorus-containing organic chemicals (organophosphates) that disrupt the mechanism by which nerves transfer messages to organs. The disruption is caused by blocking acetylcholinesterase, an enzyme that normally relaxes the activity of acetylcholine, a neurotransmitter. ...--------- --- -·---- - --- -·-- --- --- Contents • 1 Overview • 2 Biological Effects • 2.1 Mechanism of Action • 2.2 Antidotes • 3 Classes • 3.1 G-Series • 3.2 V-Series • 3.3 Novichok Agents • 3.4 Insecticides • 4 History • 4.1 The Discovery ofNerve Agents • 4.2 The Nazi Mass Production ofTabun • 4.3 Nerve Agents in Nazi Germany • 4.4 The Secret Gets Out • 4.5 Since World War II • 4.6 Ocean Disposal of Chemical Weapons • 5 Popular Culture • 6 References and External Links --------------- ----·-- - Overview As chemical weapons, they are classified as weapons of mass destruction by the United Nations according to UN Resolution 687, and their production and stockpiling was outlawed by the Chemical Weapons Convention of 1993; the Chemical Weapons Convention officially took effect on April 291997. Poisoning by a nerve agent leads to contraction of pupils, profuse salivation, convulsions, involuntary urination and defecation, and eventual death by asphyxiation as control is lost over respiratory muscles. -
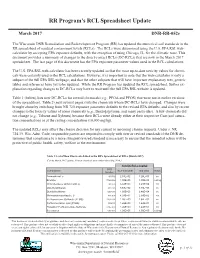
RR Program's RCL Spreadsheet Update
RR Program’s RCL Spreadsheet Update March 2017 RR Program RCL Spreadsheet Update DNR-RR-052e The Wisconsin DNR Remediation and Redevelopment Program (RR) has updated the numerical soil standards in the August 2015 DNR-RR- 052b RR spreadsheet of residual contaminant levels (RCLs). The RCLs were determined using the U.S. EPA RSL web- calculator by accepting EPA exposure defaults, with the exception of using Chicago, IL, for the climatic zone. This documentThe U.S. provides EPA updateda summary its Regionalof changes Screening to the direct-contact Level (RSL) RCLs website (DC-RCLs) in June that2015. are To now reflect in the that March 2017 spreadsheet.update, the The Wisconsin last page ofDNR this updated document the has numerical the EPA exposuresoil standards, parameter or residual values usedcontaminant in the RCL levels calculations. (RCLs), in the Remediation and Redevelopment program’s spreadsheet of RCLs. This document The providesU.S. EPA a RSL summary web-calculator of the updates has been incorporated recently updated in the Julyso that 2015 the spreadsheet.most up-to-date There toxicity were values no changes for chemi - cals madewere certainlyto the groundwater used in the RCLs,RCL calculations. but there are However, many changes it is important in the industrial to note that and the non-industrial web-calculator direct is only a subpartcontact of the (DC) full RCLsEPA RSL worksheets. webpage, Tables and that 1 andthe other 2 of thissubparts document that will summarize have important the DC-RCL explanatory changes text, generic tablesfrom and the references previous have spreadsheet yet to be (Januaryupdated. -

Safe Methods of Use 14: HSNO Class 6.1 Acutely Toxic Compounds
Safe Methods of Use 14: HSNO Class 6.1 Acutely Toxic Compounds Purpose: This applies to principal investigators (PIs), sector managers, designated laboratory person (DLPs), technical staff and students who use laboratories within the University of Auckland. Note: the word ‘shall denotes a mandatory requirement and the word ‘should’ denotes a recommendation. HSNO Class 6.1 Toxic Compounds will cover a wide range of chemicals, for which an exhaustive list cannot be supplied. Always consult MSDS sheets prior to handling any chemical, observe precautions and follow the recommendations for their handling. The mandatory recommendations in the SMOU will apply to HSNO Class 6.1 A and B compounds and should be treated as recommendations for handling HSNO Class 6.1C compounds where this appropriate. MSDS databases (Gold FFX) is available via the LEARN Database Appendix 1 provides a list (albeit not exhaustive) of HSNO 6.1 A, B and C Acutely Toxic Compounds with their classifications for your guidance. Please also note that chemicals that have primary HSNO classification of Class 3, 4, 5 or 8 may also be toxic. A. Incompatibilities Care should be taken to keep HSNO Class 6.1A and B compounds well away from liquid acids, bases, strongly oxidising solutions and reactive compounds. Safe Methods of Use 14 – HSNO Class 6.1 Acute Toxics Version 7 Feb 2019 Page 1 of 9 B. Storage 1. Containers with toxic compounds with an oral LD50 less than 5 milligrams/kg (HSNO 6.1A), shall be clearly labelled with identity of compound and a warning indicating their toxicity. 2. -

Supplement to All Hazards Receipt Facility (AHRF) Screening Protocol
EPA/600/R-10/155 December 2010 Supplement to All Hazards Receipt Facility (AHRF) Screening Protocol SCIENCE Office of Research and Development National Homeland Security Research Center Supplement to All Hazards Receipt Facility (AHRF) Screening Protocol December 2010 Office of Research and Development National Homeland Security Research Center Acknowledgments This document is intended to be supplementary to the U.S. Environmental Protection Agency (EPA) and U.S. Department of Homeland Security (DHS) September 2008 All Hazards Receipt Facility Protocol (AHRF Protocol), and attempts to address considerations raised by stakeholders since publication of the protocol. Development of this document was funded by the U.S. Environmental Protection Agency (EPA) National Homeland Security Research Center (NHSRC), and includes information provided by EPA Regions 1, 6, and 10; EPA Office of Radiation and Indoor Air (ORIA), the Association of Public Health Laboratories (APHL): State Public Health Laboratories of Connecticut, Delaware, Massachusetts, Minnesota, New Jersey, New York, and Virginia; and New York City; and the Canadian Defence Research and Development Laboratory. This document was prepared by CSC under Contract EP-W-06- 046. Disclaimer This document is intended to be supplementary to the guidance provided in the U.S. Environmental Protection Agency (EPA) and U.S. Department of Homeland Security (DHS) September 2008 All Hazards Receipt Facility Protocol (AHRF Protocol), and attempts to address considerations raised by stakeholders since publication of the protocol. This supplement assumes that: • The September 2008 AHRF Protocol was developed and provided as a guide; implementation of the protocol and the screening equipment included in the protocol may vary among locations, depending on the goals and capabilities of the laboratory to which the facility is attached. -

New Compositions Containing Carbon and Sulfur
71 New Compositions Containing Carbon and Sulfur Collin Galloway Final Seminar March 30, 1994 Because binary phases are particularly fundamental to chemistry and materials sci ence, most have been rather heavily studied, especially those that might afford molecular species. Nonetheless, compositions of the type CnSm have been overlooked and remain a promising source of new binary molecular and polymeric materials [l]. The known molecu lar architectures based on carbon and sulfur are quite varied although they are constructed from only C=C, C=S, and S-S bonds [2]. Using the zinc salt (NBu4)2[Zn(a-C3S5)2], pure [C3Ss]n was prepared via its oxida tion with sulfuryl chloride [3]. Raman and reactivity studies indicate that [C3S sln is probably polymeric although it can be converted to the dimer C6S 10 by treatment with CS2 [4]. s SAS / SIS~ s ... s~s s s>= H s=< s ~ sI +s s+n Treatment of the above [Zn(a-C3S5)2]2- with HCl gave the dithiol, C3S5H2. Like cis dimercaptoethylene [5], this alkene dithiol is unstable with respect to loss of H2S. Thermoly sis of the dithiol afforded the new binary carbon sulfide C6S8 as an insoluble red-brown powder. Both C6S 10 and C6S8 were converted to dicarbonyl derivatives through an Hg2+ pro moted 0 for S exchange of the thiocarbonyl groups. For C6S10, this reaction was accompa nied by a skeletal rearrangement of the central l,2,5,6-S4C4 ring to a 1,2,3,6 isomer as shown by single crystal X-ray diffraction. When a mixture of the previously reported [6] carbon sulfides C6S12 and C6Ss was subjected to the same 0 for S exchange conditions, only the compound C3S70 was isolated. -

NASTTPO CFATS Information 2Nd Edition
National Association of SARA Title III Program Officials Concerned with the Emergency Planning and Community Right-to-Know Act January 13, 2008 Issue 07-01 2nd Edition The Department of Homeland Security has adopted 6 CFR Part 27, a new regulation mandated by Congress, known as Chemical Facility Anti-Terrorism Standards (CFATS). The regulation is intended to fill a security gap in our country’s anti-terrorism efforts by identifying and improving the security of chemicals that are potentially at a high level of risk for release, theft, or sabotage. LEPCs and SERCs should alert EPCRA & RMP reporting facilities about these requirements. No reports are due to the LEPCs and SERCs under these requirements; however, given the potential for security requirements to have an impact on facility access for emergency responders and on emergency plans, it is critical for local planners, responders and facilities to communicate in order for a facility to satisfy the regulatory requirements. In order to aid LEPCs, SERCs and facilities in understanding these new requirements we have prepared some short-hand aids. Following this cover page is a key issue comparison between EPCRA, RMP and the CFATS regulation. As requirements may change the user is counseled to look for updated information. Following the side-by-side comparison we have edited the EPA “List of Lists” to add the Appendix A list of chemicals and thresholds from the CFATS program. This list may change and we will update these materials when that happens. The initial requirement for a facility with an Appendix A chemical at or over the threshold is to submit a Top-Screen. -

Graphical Arrays of Chemical-Specific Health Effect Reference Values for Inhalation Exposures
EPA/600/R-09/061 Graphical Arrays of Chemical-Specific Health Effect Reference Values for Inhalation Exposures Includes Errata Sheet created on 4/6/2010 September 2009 U.S. Environmental Protection Agency Office of Research and Development National Center for Environmental Assessment Research Triangle Park, NC DISCLAIMER This document has been prepared by staff from the Hazardous Pollutant Assessment Group, National Center for Environmental Assessment, U.S. Environmental Protection Agency. Any opinions, findings, conclusions, or recommendations are those of the authors and do not necessarily reflect the views of the EPA. For questions concerning this document, please contact Dr. George Woodall (919-541-3896; [email protected]). September 2009 ii Errata Sheet Created 4/6/2010 for the document titled Graphical Arrays of Chemical-Specific Health Effect Reference Values for Inhalation Exposures, Final Table or Page Erratum Figure 133 Changed “values” to “value,” deleted “and OSHA,” and added “OSHA PEL” before “ACGIH ” in the first sentence of the second paragraph. Added the following sentence at the end of the second paragraph: “It should also be noted that the original documentaion for the OSHA PEL cited it as a Ceiling Value (OSHA, 1996, 192249) but OSHA later clarified in a memo that the value was a time-weighted average (OSHA, 1996, 598129)” Figure 133 Replaced Figure 2.15 2.15 Table 137 Replaced “OSHA-Ceiling” with “OSHA-PEL (TWA)” in the first 2.15 column of Table 2.15. Replaced “10 min” with “8 hr TWA” in the second column. Added reference in last column. 140 Added reference “OSHA (1996).