Radical Scavenging and Anti-Inflammatory Activities Of
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-
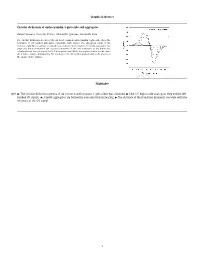
Graphical Abstract Circular Dichroism of Anthocyanidin 3-Glucoside Self
Graphical abstract Circular dichroism of anthocyanidin 3-glucoside self-aggregates Raquel Gavara, Vesselin Petrov, Alexandre Quintas, Fernando Pina * The circular dichroism spectra of the six most common anthocyanidin 3-glucoside show the formation of left handed aggregates compatible with dimers. The absorption bands of the monomer split by increasing concentration according to the formation of H and J aggregates. The angle and distance between the transition moments of the two monomers in the dimer was calculated from the splitting of the 0–0 absorption band. While the angle is similar for the series the distance changes dramatically. The intensity of the CD signal is proportional to the inverse of the square of the distance. Highlights Q10 " The circular dichroism spectra of six common anthocyanins 3-glucosides was obtained. " Like 3,5-diglucoside analogous they exhibit left- handed CD signals. " J and H aggregates are formed by concentration increasing. " The distance of the transition moments correlate with the intensity of the CD signal. 1 1 2 Circular dichroism of anthocyanidin 3-glucoside self-aggregates a a b a,⇑ 3 Q1 Raquel Gavara , Vesselin Petrov , Alexandre Quintas , Fernando Pina 4 a REQUIMTE, Departamento de Química, Faculdade de Ciências e Tecnologia, Universidade Nova de Lisboa, 2829516 Monte de Caparica, Portugal 5 b Instituto Superior de Ciencias da Saude Egas Moniz, Centro de Investigação Interdisciplinar Egas Moniz, P-2829511 Monte de Caparica, Caparica, Portugal 6 7 article info a b s t r a c t 1 9 10 Self-association constants for the flavylium cations of the six most common anthocyanidin 3-glucosides 20 11 were determined by circular dichroism (CD) and UV–Vis spectroscopy. -

Effects of Anthocyanins on the Ahr–CYP1A1 Signaling Pathway in Human
Toxicology Letters 221 (2013) 1–8 Contents lists available at SciVerse ScienceDirect Toxicology Letters jou rnal homepage: www.elsevier.com/locate/toxlet Effects of anthocyanins on the AhR–CYP1A1 signaling pathway in human hepatocytes and human cancer cell lines a b c d Alzbeta Kamenickova , Eva Anzenbacherova , Petr Pavek , Anatoly A. Soshilov , d e e a,∗ Michael S. Denison , Michaela Zapletalova , Pavel Anzenbacher , Zdenek Dvorak a Department of Cell Biology and Genetics, Faculty of Science, Palacky University, Slechtitelu 11, 783 71 Olomouc, Czech Republic b Institute of Medical Chemistry and Biochemistry, Faculty of Medicine and Dentistry, Palacky University, Hnevotinska 3, 775 15 Olomouc, Czech Republic c Department of Pharmacology and Toxicology, Charles University in Prague, Faculty of Pharmacy in Hradec Kralove, Heyrovskeho 1203, Hradec Kralove 50005, Czech Republic d Department of Environmental Toxicology, University of California, Meyer Hall, One Shields Avenue, Davis, CA 95616-8588, USA e Institute of Pharmacology, Faculty of Medicine and Dentistry, Palacky University, Hnevotinska 3, 775 15 Olomouc, Czech Republic h i g h l i g h t s • Food constituents may interact with drug metabolizing pathways. • AhR–CYP1A1 pathway is involved in drug metabolism and carcinogenesis. • We examined effects of 21 anthocyanins on AhR–CYP1A1 signaling. • Human hepatocytes and cell lines HepG2 and LS174T were used as the models. • Tested anthocyanins possess very low potential for food–drug interactions. a r t i c l e i n f o a b s t r a c t -

Supplementary Materials
Supplementary Materials Table S1. Metals with stdev W1 W2 W3 W4 W5 W6 W7 W8 W9 W10 W11 W12 W13 As <LOD <LOD 19.84±0.03 <LOD <LOD <LOD <LOD <LOD <LOD <LOD <LOD <LOD <LOD Ba 32.6±0.3 33.8±0.4 26.1±0.2 123.3±0.9 79.8±0.8 79.1±0.7 83.3±0.6 239.7±0.8 58.1±0.4 77.7±0.3 32.1±0.3 31.7±0.5 122.1±0.8 Cd 0.365±0.004 1.553±0.002 0.939±0.002 1.254±0.003 0.386±0.005 1.367±0.008 0.733±0.004 0.050±0.002 0.976±0.002 1.644±0.006 <LOD 0.593±0.004 0.375±0.003 Co 3.64±0.04 2.09±0.02 0.98±0.03 4.40±0.06 2.77±0.02 1.83±0.02 2.42±0.02 2.68±0.03 7.34±0.09 7.78±0.07 2.12±0.04 1.61±0.02 2.60±0.03 Cr <LOD <LOD <LOD <LOD 4.322±0.005 3.030±0004 <LOD 6.417±0.006 15.41±0.02 14.82±0.05 11.12±0.04 9.15±0.02 3.111±0.004 Pb <LOD 56.0±0.8 <LOD 20.8±0.9 80±2 11.7±0.6 2.20±0.05 175±3 45.0±0.8 <LOD 0.830±0.007 0.538±0.006 14.9±0.3 Sb 32.8±0.5 8.59±0.09 19.1±0.2 18.±0.2 67.5±0.5 37.9±0.2 9.58±0.05 46.5±0.3 44.8±0.4 18.50±0.08 7.26±0.03 44.3±0.2 29.6±0.4 Se <LOD <LOD <LOD <LOD 1.45±0.09 0.30±0.05 0.23±0.08 25.7±0.4 2.87±0.2 2.06±0.04 2.91±0.8 2.38±0.07 2.99±0.06 Ni 4.17±0.05 46.0±0.2 0.569±0.005 30.9±0.3 15.3±0.2 5.77±0.02 35.2±0.3 13.86±0.04 50.6±0.7 17.9±0.2 9.99±0.03 0.197±0.007 5.00±0.02 Al 566±5 260.1±0.5 155.3±0.4 720±5 447±4 290.7±2 26.5±0.4 155.4±0.9 444±4 329±3 1027±10 567.7±8 236±3 Cu 97.5±0.8 469±3 36.3±0.9 10.07±0.05 <LOD <LOD <LOD <LOD 21.19±0.07 7.73±0.03 0.813±0.05 49.3±0.8 50.2±1.0 Mn 735±30 613±10 642±6 1016±6 762±3 743±5 839±8 1634±30 1732±20 1472±20 887±10 771±6 1299±10 V 4.18±0.02 1.894±0.007 0.574±0.002 <LOD 1.530±0.005 <LOD 1.948±0.006 3.179±0.004 3.235±0.007 -

Effect of Different Anthocyanidin Glucosides on Lutein Uptake By
molecules Article Effect of Different Anthocyanidin Glucosides on Lutein Uptake by Caco-2 Cells, and Their Combined Activities on Anti-Oxidation and Anti-Inflammation In Vitro and Ex Vivo Minh Anh Thu Phan 1,*, Martin Bucknall 2 and Jayashree Arcot 1,* ID 1 Food and Health Cluster, School of Chemical Engineering, UNSW, Sydney, NSW 2052, Australia 2 Mark Wainwright Analytical Centre, UNSW, Sydney, NSW 2052, Australia; [email protected] * Correspondence: [email protected] (M.A.T.P.); [email protected] (J.A.); Tel.: +61-2-9385-5360 (J.A.) Academic Editors: Natália Martins and Derek J. McPhee Received: 23 July 2018; Accepted: 11 August 2018; Published: 14 August 2018 Abstract: The interactive effects on anti-oxidation and anti-inflammation of lutein combined with each of the six common anthocyanidin glucosides were studied in both chemical and cellular systems. The combined phytochemicals showed an antagonism in the inhibition of lipid oxidation in a liposomal membrane, but showed an additive effect on cellular antioxidant activity in Caco-2 cells. Lutein was an active lipoxygenase inhibitor at 2–12 µM while anthocyanins were inactive. The concentration of lutein when it was used in combination with anthocyanins was 25–54% higher than when lutein was used alone (i.e., IC50 = 1.2 µM) to induce 50% of lipoxygenase inhibition. Only the combination of lutein with malvidin-3-glucoside showed anti-inflammatory synergy in the suppression of interleukin-8, and the synergy was seen at all three ratios tested. Some mixtures, however, showed anti-inflammatory antagonism. The presence of anthocyanins (5–7.5 µM) did not affect lutein uptake (2.5–5 µM) by Caco-2 cells. -

In Primary Human Hepatocytes
Food & Function Accepted Manuscript This is an Accepted Manuscript, which has been through the Royal Society of Chemistry peer review process and has been accepted for publication. Accepted Manuscripts are published online shortly after acceptance, before technical editing, formatting and proof reading. Using this free service, authors can make their results available to the community, in citable form, before we publish the edited article. We will replace this Accepted Manuscript with the edited and formatted Advance Article as soon as it is available. You can find more information about Accepted Manuscripts in the Information for Authors. Please note that technical editing may introduce minor changes to the text and/or graphics, which may alter content. The journal’s standard Terms & Conditions and the Ethical guidelines still apply. In no event shall the Royal Society of Chemistry be held responsible for any errors or omissions in this Accepted Manuscript or any consequences arising from the use of any information it contains. www.rsc.org/foodfunction Page 1 of 23 Food & Function Effects of anthocyans on the expression of organic anion transporting polypeptides ( SLCOs /OATPs) in primary human hepatocytes Juliane Riha,a Stefan Brenner,a Alzbeta Srovnalova, b Lukas Klameth,c Zdenek Dvorak, b Walter Jäger a and Theresia Thalhammer*d Affiliations: a Department of Clinical Pharmacy and Diagnostics, University of Vienna, Vienna, Austria b Department of Cell Biology and Genetics, Faculty of Science, Palacky University, Olomouc, Czech Manuscript Republic c Ludwig Boltzmann Society, Cluster for Translational Oncology, Vienna, Austria d Department of Pathophysiology and Allergy Research, Center of Pathophysiology, Medical University of Vienna, Vienna, Austria, Running Title: Accepted Effects of anthocyans on expression of OATPs in primary human hepatocytes Corresponding Author: Dr. -

(12) Patent Application Publication (10) Pub. No.: US 2011/0268825 A1 Burgos Et Al
US 20110268825A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2011/0268825 A1 Burgos et al. (43) Pub. Date: Nov. 3, 2011 (54) COMPOSITIONS THAT INCLUDE A6IP3L/04 (2006.01) ANTHOCYANDINS AND METHODS OF USE A6IP3L/00 (2006.01) A6IR 36/45 2006.O1 (76) Inventors: Rafael Burgos, Valdivia (CL); Juan A6IP 29/00 308: Hancke, Region Metropolitana A6IP3/00 (2006.01) (CL): Evelyn Jara, Valdivia (CL): A6IP3/04 (2006.01) Maria Hidalgo, Valdivia (CL) A6IP3/10 (2006.01) A63L/352 2006.O1 (21) Appl. No.: 13/076,117 A6IP35/00 308: (22) Filed: Mar. 30, 2011 A6IP3L/2 (2006.01) (52) U.S. Cl. .......................... 424/732; 424/777: 514/456 Related U.S. Application Data (63) Continuation of application No. PCT/IB2010/002698, (57) ABSTRACT filed on Oct. 21, 2010. The compositions described herein and featured in the (60)60) Provisional app lication1cauon No. 91/453.833,61/253.835, filedIlled on Oct9c. presentcombinations invention rich include in delphinidins, those that compriseincluding anthocyanidin delphinidins 21, 2009, provisional application No. 61/279.541, Such as the ones found in berries. The compositions can filed on Oct. 22, 2009. optionally include either compositions that comprise O O andrographolides, such as the ones found in a plant of the Publication Classification genus Andrographis, or compositions that comprise combi (51) Int. Cl. nations of myrtillin, quercetin, or caffeoylquinic derivatives A6 IK 36/18 (2006.01) and proanthocyanidins. Such as the ones found in the herba of A6IP37/00 (2006.01) a plant of the genus Vaccinium. Patent Application Publication Nov. -

Bbm:978-3-540-73733-9/1.Pdf
Sachverzeichnis Abelmoschus esculenta 481 Aflatoxine 449 Abelmoschus esculentus 491 Afzelechin 469 Abietinsäure 26 Agar-Agar 362, 402, 412 Acacetin 478 Agarose 412, 413 Acacia 402 Agave sisalana 69, 70 Acanthoscelides obtectus 648, 649 Aglycon 353 Acarbose 372, 399, 400, 401 Agmatin 151 ACE 292 Agnosterin 34 Acetoacetyl-ACP 575 AIDS 410 Acetobacter suboxidans 375 Ajmalicin 200 α-Acetobromglucose 355 Ajmalin 196 Acetoide 5, 43 Ajuga ripponesis 391 Acetylcholin 143ff, 148, 158, 185, 323 Ajugose 391 Acetylcholinesterase 323 Akazie 478, 481 Acetylcoenzym A 44, 376, 378, 442 aktivierte Isopren 44 N-Acetyl-Cystein 265 Alanin 254, 270, 273f, 276, 288, 315 N-Acetyl-D-glucosamin 363 β-Alanin 259 β-Acetyldigoxin 112 Albumin 308 N-Acetylmuraminsäure 363, 365 Albumine 312 O-Acetylsamandarin 234 Alcaligenes faecalis 402 Achras zapota 60 Aldonsäuren 342 Aciclovir 439 Aldosen 335 Aconitum 1 Aldosteron 76, 85, 87 ACP 575 Algin 402 Acrinomycin 260 Alginate 402, 413 Actinomadura 168 Alitam 307 Actinomycin D 306 Alkaloide 143 Actinoplanes 399 Allelopathica 180 Acyl-Carrierprotein 575 Alliin 261 Adamantoylchlorid 352 Allithiamin 426 ADD 95 Allomethylose 341 Adenin 425, 426, 430, 436, 452 Aloin 386 Adenocalymna alliaceum 478 Amanita muscaria 148 Adenosin 432 Amanita phalloides 329 Adenosintriphosphat 442 Amanita strobiliformis 261 Adhatoda vasica 192 Amanitin 329 Adonis vernalis 100, 343 Amantia mappa 148 Adonitol 343 Amaranthenol 41 Adrenalin 145, 146, 147 Amaranthöl 34, 41 Adriamycin 293, 604 AMD 40 676 Sachverzeichnis Amentoflavon 479 anomeren Effekt 339 -

Table S1. Phytochemicals Identified from Different Pomegranate Tissues
1 Table S1. Phytochemicals identified from different pomegranate tissues. The chemical structures, 2 molecular formulas, and molecular weights of the phytochemicals are shown. The analytical methods, 3 tissues of identification, and representative references are also indicated. CAS, chemical abstracts 4 service; DAD, diode array detection; ESR, electron spin resonance; FD, fluorescence detection; FID, 5 flame ionization detection; ID, identification method; IR, infrared spectroscopy; MP, melting point; 6 MS, mass spectrometry; MW, molecular weight; NMR, nuclear magnetic resonance; TLC, thin layer 7 chromatography. 8 Molecules 2017, 22, x; doi: FOR PEER REVIEW www.mdpi.com/journal/molecules Molecules 2017, 22, x FOR PEER REVIEW 2 of 20 Name Structure Formula MW ID Tissue References Ellagitannins, gallotannins and derivatives Brevifolin C12H8O6 248.1900 NMR Leaf [1] Brevifolin carboxylic acid C13H8O8 292.1990 NMR Leaf, flower, [1-3] heartwood Brevifolin carboxylic acid 10- C13H7KO11S 410.3463 NMR Leaf [4] monopotassium sulphate Castalagin C41H26O26 934.6330 NMR Stem bark [5] Casuariin C34H24O22 784.5440 NMR Stem bark [5] Casuarinin C41H28O26 936.6490 NMR Peel, stem bark [5, 6] R1 = Galloyl (β-configuration), R2 = R3 = H Corilagin C27H22O18 634.4550 NMR Peel, leaf [6, 7] R1 = Galloyl (α-configuration), R2 = R3 = H Isocorilagin C27H22O18 634.4550 NMR Flower [8] R2 = Galloyl, R1 = R3 = H Hippomanin A C27H22O18 634.4550 IR Flower [2] R1 = R2 = H, R3 = Galloyl Gemin D C27H22O18 634.4550 IR Flower [2] Diellagic acid rhamnosyl(1→4) C40H30O24 894.6560 -

PHYTOPLAN ® Pflanzliche Wirkstoffe Und Analytik I
Version 4/2013 PHYTOPLAN ® Pflanzliche Wirkstoffe und Analytik I Product List 2013 Reference Substances Natural Compounds PHYTO PLAN Diehm & Neuberger GmbH Im Neuenheimer Feld 515 D-69120 Heidelberg Germany Phone: 0049 62 21 / 40 13 47 Fax: 0049 62 21 / 43 76 64 e-Mail: [email protected] Internet: www.Phytoplan.com PHYTO PLAN Dear customer, We are pleased to introduce our new catalogue for the year 2013. Therein you will find many new products and also a greater range in the qualities of the compounds differing in the degree of purity and the documents delivered. Please decide which item is the proper for your purpose. Catalogue of natural compounds In our catalog we have listed the substances which are near-term available. Often you can choose a definite degree of purity and extent of documentation (see column ' documents delivered '). The substances are mostly of high purity and are available as identification standards or HPLC standards dependent on the extent of the documentation. Some compounds are offered also in larger quantities with a lower degree of purity. All substances are delivered with an individual certificate of analysis which shows the purity by means of HPLC DAD and DAD ultraviolet spectrum. Due to their purity (usually >98.0 – 99.0 %) the reference substances in our catalogue are suitable for ambitious applications. On customer's request the range of the current documentation can be individually expanded and adapted. Please check which specific requirement of the documentation for your application (e.g. for authorization or registration, HPLC standard, working standard etc.) is demanded. -
PHYTOPLAN ® Pflanzliche Wirkstoffe Und Analytik I
Version 2/2016 PHYTOPLAN ® Pflanzliche Wirkstoffe und Analytik I Product List 2016 Reference Substances Natural Compounds PHYTO PLAN Diehm & Neuberger GmbH Im Neuenheimer Feld 515 D-69120 Heidelberg Germany Phone: 0049 62 21 / 40 13 47 Fax: 0049 62 21 / 43 76 64 e-Mail: [email protected] Internet: www.Phytoplan.com PHYTO PLAN Dear customer, We are pleased to introduce our new catalogue for the year 2016. Therein you will find many new products and also a greater range in the qualities of the compounds differing in the degree of purity and the documents delivered. Please decide which item is the proper for your purpose. Catalogue of natural compounds In our catalog we have listed the substances which are near-term available. Often you can choose a definite degree of purity and extent of documentation (see column ' documents delivered '). The substances are mostly of high purity and are available as identification standards or HPLC standards dependent on the extent of the documentation. Some compounds are offered also in larger quantities with a lower degree of purity. All substances are delivered with an individual certificate of analysis which shows the purity by means of HPLC DAD and DAD ultraviolet spectrum. Due to their purity (usually >97.0 – 99.0 %) the reference substances in our catalogue are suitable for ambitious applications. On customer's request the range of the current documentation can be individually expanded and adapted. Please check which specific requirement of the documentation for your application (e.g. for authorization or registration, HPLC standard, working standard etc.) is demanded. -
Reference Substances 2018/2019
Reference Substances 2018 / 2019 Reference Substances Reference 2018/2019 Contents | 3 Contents Page Welcome 4 Our Services 5 Reference Substances 6 Index I: Alphabetical List of Reference Substances and Synonyms 156 Index II: Plant-specific Marker Compounds 176 Index III: CAS Registry Numbers 214 Index IV: Substance Classification 224 Our Reference Substance Team 234 Order Information 237 Order Form 238 Prices insert 4 | Welcome Welcome to our new 2018 / 2019 catalogue! PhytoLab proudly presents the new you will also be able to view exemplary Index I contains an alphabetical list of all 2018 / 2019 catalogue of phyproof® certificates of analysis and download substances and their synonyms. It pro- Reference Substances. The seventh edition material safety data sheets (MSDS). vides information which name of a refer- of our catalogue now contains well over ence substance is used in this catalogue 1300 phytochemicals. As part of our We very much hope that our product and guides you directly to the correct mission to be your leading supplier of portfolio meets your expectations. The list page. herbal reference substances PhytoLab of substances will be expanded even has characterized them as primary further in the future, based upon current If you are a planning to analyse a specific reference substances and will supply regulatory requirements and new scientific plant please look for the botanical them together with the comprehensive developments. The most recent information name in Index II. It will inform you about certificates of analysis you are familiar will always be available on our web site. common marker compounds for this herb. with. -

Signature Redacted for Privacy
AN ABSTRACT OF THE DISSERTATION OF Jungmin Lee for the degree of Doctor of Philosophy in Food Science and Technology presented January 13. 2004. Title: The Blueberry: Composition Anthocyanins. And Polyphenolics. Abstract approved: Signature redacted for privacy. .4 Ronald E. Wroistad Frozen blueberries were pilot-plant-processed into juice and concentrate by 2 treatments (heat and SO2), and a control. Qualitative, and quantitative anthocyanin and polyphenolic content were monitored after each processing step. Major losses occurred after the initial processing steps. Juice processing by-product contained a substantial amount of anthocyanins and polyphenolics (>42% and >15%, respectively of the starting material), which indicated they remained a good source for further extraction. Commercial juice processing enzymes (n=9) and other extraction methods were evaluated for extraction of anthocyanins and dietary antioxidants from blueberry skins and wholeberries on a bench-top scale. Blueberries were manually separated into peels, flesh, and seeds. The fractions were evaluated for (qualitative and quantitative) anthocyanin (ACY) and polyphenolic (TP) content, and antioxidant capacity. Blueberry skins had the greatest antioxidant capacity .with the highest ACY and TP content, followed by flesh, and then seeds. The polyphenolic profiles were different in each of the three fractions. High performance liquid chromatography (HPLC), electrospray mass spectrometry (ES-MS), and liquid chromatography mass spectrometry (LC-DAD-MS) were used in identifying the individual anthocyanins. LC-DAD-MS was an invaluable tool in identification of the individual anthocyanins. Four Pacific Northwest native huckleberries (n>38 populations) were collected to compare their ACY and TP content to 'Rubel', which is an important criterion for breeding programs or commercialization.