In Primary Human Hepatocytes
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
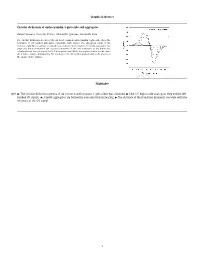
Graphical Abstract Circular Dichroism of Anthocyanidin 3-Glucoside Self
Graphical abstract Circular dichroism of anthocyanidin 3-glucoside self-aggregates Raquel Gavara, Vesselin Petrov, Alexandre Quintas, Fernando Pina * The circular dichroism spectra of the six most common anthocyanidin 3-glucoside show the formation of left handed aggregates compatible with dimers. The absorption bands of the monomer split by increasing concentration according to the formation of H and J aggregates. The angle and distance between the transition moments of the two monomers in the dimer was calculated from the splitting of the 0–0 absorption band. While the angle is similar for the series the distance changes dramatically. The intensity of the CD signal is proportional to the inverse of the square of the distance. Highlights Q10 " The circular dichroism spectra of six common anthocyanins 3-glucosides was obtained. " Like 3,5-diglucoside analogous they exhibit left- handed CD signals. " J and H aggregates are formed by concentration increasing. " The distance of the transition moments correlate with the intensity of the CD signal. 1 1 2 Circular dichroism of anthocyanidin 3-glucoside self-aggregates a a b a,⇑ 3 Q1 Raquel Gavara , Vesselin Petrov , Alexandre Quintas , Fernando Pina 4 a REQUIMTE, Departamento de Química, Faculdade de Ciências e Tecnologia, Universidade Nova de Lisboa, 2829516 Monte de Caparica, Portugal 5 b Instituto Superior de Ciencias da Saude Egas Moniz, Centro de Investigação Interdisciplinar Egas Moniz, P-2829511 Monte de Caparica, Caparica, Portugal 6 7 article info a b s t r a c t 1 9 10 Self-association constants for the flavylium cations of the six most common anthocyanidin 3-glucosides 20 11 were determined by circular dichroism (CD) and UV–Vis spectroscopy. -

(Roxb.) Craib and Kadsura Coccinea (Lem.) AC
foods Article Phenolic Profiles, Antioxidant, and Inhibitory Activities of Kadsura heteroclita (Roxb.) Craib and Kadsura coccinea (Lem.) A.C. Sm. Varittha Sritalahareuthai 1, Piya Temviriyanukul 1,2 , Nattira On-nom 1,2 , Somsri Charoenkiatkul 1 and Uthaiwan Suttisansanee 1,2,* 1 Institute of Nutrition, Mahidol University, Salaya, Phuttamonthon, Nakhon Pathom 73170, Thailand; [email protected] (V.S.); [email protected] (P.T.); [email protected] (N.O.-n.); [email protected] (S.C.) 2 Food and Nutrition Academic and Research Cluster, Institute of Nutrition, Mahidol University, Salaya, Phuttamonthon, Nakhon Pathom 73170, Thailand * Correspondence: [email protected]; Tel.: +66-(0)2800-2380 (ext. 422) Received: 4 August 2020; Accepted: 31 August 2020; Published: 2 September 2020 Abstract: Kadsura spp. in the Schisandraceae family are woody vine plants, which produce edible red fruits that are rich in nutrients and antioxidant activities. Despite their valuable food applications, Kadsura spp. are only able to grow naturally in the forest, and reproduction handled by botanists is still in progress with a very low growth rate. Subsequently, Kadsura spp. were listed as endangered species by the International Union for Conservation of Nature and Natural Resources (IUCN) in 2011. Two different Kadsura spp., including Kadsura coccinea (Lem.) A.C. Sm. and Kadsura heteroclita (Roxb.) Craib, are mostly found in northern Thailand. These rare, wild fruits are unrecognizable to outsiders, and there have only been limited investigations into its biological properties. This study, therefore, aimed to comparatively investigate the phenolic profiles, antioxidant activities, and inhibitory activities against the key enzymes involved in diabetes (α-glucosidase and α-amylase) and Alzheimer’s disease (acetylcholinesterase (AChE), butyrylcholinesterase (BChE), and beta-secretase 1 (BACE-1)) in different fruit parts (exocarp, mesocarp (edible part), seed, and core) of Kadsura coccinea (Lem.) A.C. -

Alteration of Anthocyanin Glycosylation in Cranberry Through Interspecific Hybridization
J. AMER. Soc. HORT. Sci. 130(5):711-715. 2005. Alteration of Anthocyanin Glycosylation in Cranberry Through Interspecific Hybridization Nicholi Vorsa Philip E. Marucci Center for Blueberry and Cranberry Research and Extension, Rutgers University, 125A Lake Oswego Road, Chatsworth, NJ 08019 James J. Polashock1 USDA—ARS Fruit Lab, 125A Lake Oswego Road, Chatsworth, NJ 08019 ADDITIONAL INDEX WORDS. Vaccinium macrocarpon, Vaccinium oxycoccus, antioxidant, bioavailability, flavonoid ABSTRACT. The flavonoids of american cranberry (Vaccinium macrocarpon Alt.) are documented to be beneficial for hu- man health. Among their benefits is a high antioxidant potential, with anthocyanin glycosides being the main contribu- tors. Flavonoid glucose conjugates are reported to be more bioavailable than those with other sugar conjugates. The anthocyanin glycosides of V. macrocarpon fruit are mainly galactosides and arabinosides of the aglycones, cyanidin and peonidin, with less than 8% glucosides. In contrast, the fruit anthocyanins of another cranberry species, V. oxycoccus L. were found to be largely glucosides of cyanidin and peonidin. Interspecific hybrids between these two species were intermediate to the parental species in the proportion of fruit anthocyanin glucosides. About half the progeny (1:1 segregation) in a backcross population (to V. macrocarpon) maintained the relatively high anthocyanin glucoside ratio. In this study, we demonstrate the genetic manipulation of anthocyanin glycosylation in cranberry using interspecific hybridization, resulting in dramatically increased glucose-conjugated anthocyanins. Flavonoids are considered to be secondary metabolites, which The cultivated american cranberry (V. macrocarpon) is recog- have been associated with roles in ultraviolet protection, plant nized for its brilliant red fruit due to an abundance of anthocyanins sexual reproduction, pollinator attraction, symbiotic plant—microbe in the fruit epidermal tissues. -

(Lapageria Rosea) C
J. Chil. Chem. Soc., 54, Nº 2 (2009) ANTHOCYANINS THAT CONFER CHARACTERISTIC COLOR TO RED COPIHUE FLOWERS (LAPAGERIA ROSEA) C. VERGARA1, D. VON BAER1*, I. HERMOSÍN2, A. RUIZ1, M.A. HITSCHFELD1, N. CASTILLO2 AND C. MARDONES1. 1 Universidad de Concepción, Departamento de Análisis Instrumental, Facultad de Farmacia, Casilla 160 – C, Concepción, Chile. 2 Universidad de Castilla-La Mancha, Escuela Universitaria de Ingeniería Técnica Agrícola, Ronda de Calatrava 7, 13071 Ciudad Real, España. (Received: January 7, 2009 - Accepted: February 25, 2009) ABSTRACT The Copihue (Lapageria rosea), also known as the Chilean bellflower, is the national flower of Chile and is the only species in the genus Lapageria. The copihue’s tepals are commonly red, with white or pink being less common. The red color of the copihue has been glorified in legends, poems and popular songs. The present work studies the pigments that confer red copihues their characteristic color. The principal types of cyanidin present in red copihue’s tepals are cyanidin-3-O-rhamnosylglucoside, followed by cyanidin-3-O-glucoside, and while only the latter is detected in pink tepals and neither one are detected in white flowers. Based on the obtained results by HPLC-ESI-MSn and HPLC-DAD, it is concluded that rhamnosyl- and glucosyl-derivatives of cyanidin, which present respectively an absorption maximum at 518 and 516 nm, confer the characteristic red color to red copihues. Furthermore, glycosilated cyanidin derivatives, pigments derived from other anthocyanidins, were not detected in red copihue flowers even when they are present in other red flowering plants. Keywords: flower, copihue, Lapageria rosea, anthocyanins, cyanidin-3-O-rutinoside, cyanidin-3-O-glucoside. -

Effects of Anthocyanins on the Ahr–CYP1A1 Signaling Pathway in Human
Toxicology Letters 221 (2013) 1–8 Contents lists available at SciVerse ScienceDirect Toxicology Letters jou rnal homepage: www.elsevier.com/locate/toxlet Effects of anthocyanins on the AhR–CYP1A1 signaling pathway in human hepatocytes and human cancer cell lines a b c d Alzbeta Kamenickova , Eva Anzenbacherova , Petr Pavek , Anatoly A. Soshilov , d e e a,∗ Michael S. Denison , Michaela Zapletalova , Pavel Anzenbacher , Zdenek Dvorak a Department of Cell Biology and Genetics, Faculty of Science, Palacky University, Slechtitelu 11, 783 71 Olomouc, Czech Republic b Institute of Medical Chemistry and Biochemistry, Faculty of Medicine and Dentistry, Palacky University, Hnevotinska 3, 775 15 Olomouc, Czech Republic c Department of Pharmacology and Toxicology, Charles University in Prague, Faculty of Pharmacy in Hradec Kralove, Heyrovskeho 1203, Hradec Kralove 50005, Czech Republic d Department of Environmental Toxicology, University of California, Meyer Hall, One Shields Avenue, Davis, CA 95616-8588, USA e Institute of Pharmacology, Faculty of Medicine and Dentistry, Palacky University, Hnevotinska 3, 775 15 Olomouc, Czech Republic h i g h l i g h t s • Food constituents may interact with drug metabolizing pathways. • AhR–CYP1A1 pathway is involved in drug metabolism and carcinogenesis. • We examined effects of 21 anthocyanins on AhR–CYP1A1 signaling. • Human hepatocytes and cell lines HepG2 and LS174T were used as the models. • Tested anthocyanins possess very low potential for food–drug interactions. a r t i c l e i n f o a b s t r a c t -

Anthocyanin Metabolites in Human Urine and Serum
Downloaded from https://www.cambridge.org/core British Journal of Nutrition (2004), 91, 933–942 DOI: 10.1079/BJN20041126 q Minister of Public Works and Government Services Canada 2003 Anthocyanin metabolites in human urine and serum . IP address: 1,2 1,2 2 3 Colin D. Kay , G. Mazza *, Bruce J. Holub and Jian Wang 170.106.34.90 1Pacific Agri-Food Research Center, Agriculture and Agri-Food Canada, 4200 Hwy 97 Summerland, British Columbia, Canada V0H 1Z0 2 Department of Human Biology and Nutritional Sciences, University of Guelph, Ontario, Canada , on 3 Calgary Laboratory, Canadian Food Inspection Agency, Calgary, Alberta, Canada 02 Oct 2021 at 01:38:57 (Received 26 September 2003 – Revised 2 February 2004 – Accepted 11 February 2004) In the present study we investigated the metabolic conversion of cyanidin glycosides in human subjects using solid-phase extraction, HPLC–diode array detector, MS, GC, and enzymic techniques. Volunteers consumed approximately 20 g chokeberry extract containing , subject to the Cambridge Core terms of use, available at 1·3 g cyanidin 3-glycosides (899 mg cyanidin 3-galactoside, 321 mg cyanidin 3-arabinoside, 51 mg cyanidin 3-xyloside and 50 mg cyanidin 3-glucoside). Blood samples were drawn at 0, 0·5, 1, and 2 h post-consumption of the extract. Urine samples were also collected at 0, 4–5, and 22–24 h. We have confirmed that human subjects have the capacity to metabolise cyanidin 3-glycosides, as we observed at least ten individual anthocyanin metabolites in the urine and serum. Average concentrations of anthocyanins and anthocyanin metabolites in the urine reached levels of 17·9 (range 14·9–20·9) mmol/l within 5 h post-consumption and persisted in 24 h urine samples at levels of 12·1 (range 11·1–13·0) nmol/l. -

A Biochemical Survey of Some Mendelian Factors for Flower Colour
A BIOCHEMICAL 8UP~VEY OF SOME MENDELIAN FACTOI%S FO].~ FLOWEP~ COLOU~. BY ROSE SCOTT-MONCI~IEFF. (John Inncs Horticultural Institution, London.) (With One Text-figure.) CONTENTS. PAGE P~rb I. Introductory ].17 (a) The plastid 1)igmenl~s ] 21 (b) The a,n~hoxan~hius: i~heir backgromld, co-pigment and interaction effecbs upon flower-colour v~ri~bion 122 (c) The ani~hocyauins ] 25 (c) Col[oidM condition . 131 (f) Anthoey~nins as indic~bors 132 (g) The source of tim ~nl;hoey~nins 133 ]?ar[ II, Experimental 134 A. i~ecen~ investigations: (a) 2Prim,ula si,sensis 134- (b) Pa,l)aver Rhoeas 14.1 (c) Primuln aca.ulis 147 (d) Chc.l)ranth'ss Chci,rl 148 (e) ltosa lmlyanlha . 149 (f) Pelargonium zomdc 149 (g) Lalh,ymts odor~,l,us 150 (h) Vcrbom, hybrids 153 (i) Sl;'e2)loca~'])uG hybrids 15~ (j) T'rol)aeolu,m ,majors ] 55 ]3. B,eviews of published remflts of bhe t~u~horand o~hers.. (a) Dahlia variabilis (Lawreuce and Scol,~-Monerieff) 156 (b) A.nlb'rhinum majors (Wheklalo-Onslow, :Basseb~ a,nd ,~cobb- M.oncrieff ) 157 (c) Pharbilis nil (I-Iagiwam) . 158 (d) J/it& (Sht'itl.er it,lid Anderson) • . 159 (e) Zect d]f.ctys (~&udo, Miiner trod 8borl/lall) 159 Par~, III. The generM beh~wiour of Mendelian £acbors rot' flower colour . 160 Summary . 167 tLefermmes 168 I)AI~T I. II~TI~O])UOTOnY. Slm~C~ Onslow (1914) m~de the first sfudy of biochemica] chal~ges in- volved in flower-eolour va,riadon, our pro'ely chemical knowledge of bhe 118 A Bio&emical Su~'vey oI' Factor's fo~ • Flowe~' Colou~' anthocya.nin pigments has been considerably advanced by the work of Willstgtter, P~obinson, Karrer and their collaborators. -

Anthocyanin Pigments in Redbud (Cercis Spp) Flowers
Veazie et al. J Hortic Sci Res 2017, 1(1):13-18 DOI: 10.36959/745/393 | Volume 1 | Issue 1 Journal of Horticultural Science and Research Research Article Open Access Anthocyanin Pigments in Redbud (Cercis spp) Flowers Penelope Perkins-Veazie*, Guoying Ma and Dennis Werner Department of Horticultural Science, Plants for Human Health Institute, North Carolina State University, USA Abstract Redbud (Cercis spp.) is used as a spring flowering ornamental tree and is found wild in much of North America. Typically flowers are light purple although there are selected cultigens that are white, rose, or red-purple. Flowers from cultigens common to the eastern U.S. and from wild Eastern redbud (C. canadensis) were collected and tested for color and anthocyanin pigment composition. The anthocyanins cyanidin 3-glucoside, petunidin 3-glucoside, peonidin 3-glucoside, and malvidin 3-glucoside were most aboundant in purple, rose, and red-purple redbud flowers and total anthocyanin content was 2263 to 8730 mg.kg DW-1. Small amounts of delphinidin, cyanidin, and petunidin 3, diglucosides were also present. Most of the typical purple-flowered redbuds contained cyanidin 3-glucoside as the dominant pigment, while the red-purple flowered ‘Appalachian Red’ and ‘Crosswicks Red’ contained malvidin 3,5-diglucoside as the dominant anthocyanin. An unknown anthocyanin was present in all redbud flowers, and was higher in the red-purple flowered phenotypes. These results show that the color of redbud flowers is from anthocyanins, predominantly cyanidin 3-glucoside and malvidin 3,5-diglucoside, with malvidin 3,5-diglucoside as the primary pigment in red-purple flowers and cyanidin 3-glucoside dominant in purple flowers. -

Anthocyanin Biosynthesis 21421.Pdf
Anthocyanin Biosynthesis – https://www.kegg.jp/kegg-bin/highlight_pathway?scale=1.0&map=map00942&keyword=flavonoids Anthocyanidins (aglycones) and anthocyanins (glycosides) are common plant pigments and belong to a structural subclass of flavonoids characterized by a 2- phenylbenzopyrylium unit. They are derived along the flavonoid modification pathways and further separated into three types, pelargonidin, cyanidin, and delphinidin, due to the different number of hydroxyl groups in the phenyl group. (Flavonoid Biosynthesis) -> [1,2,3] 1) Pelargonidin -> anthocyanidin 3-O-glucosyltransferase -> Pelargonidin 3-O- glucoside -> [1,2,3,4,5,6,7] 1) anthocyanin 3-O-glucoside-6’’-O- malonyltransferase -> Pelargonidin 3-O-(6-O-malonyl-beta-D-glucoside) -> anthocyanidin 3-O-glucoside-3’’,6’’-O-dimalonyltransferase -> Pelargonidin 3-O-3’’,6’’-O-dimalonylglucoside OR 2) anthocyanidin 3-O-glucoside 2’’-O- glucosyltransferase -> Pelargonidin 3-O-sophoroside OR 3) Pelargonidin 3- O-rutinoside -> cyanidin 3-O-rutinoside 5-O-glucosyltransferase -> Pelargonidin 3-O-rutinoside 5-O-beta-D-glucoside OR 4) cyanidin 3-O- glucoside 7-O-glucosyltransferase (acyl-glucose) -> Pelargonidin 3,7-di-O- beta-D-glucoside OR 5) anthocyanidin 3-O-glucoside 2’’’-O- xylosyltransferase -> Pelargonidin 3-O-beta-D-sambubioside -> anthocyanin 3-O-sambubioside 5-O-glucosyltransferase -> Pelargonidin 5-O-beta-D- glucoside 3-O-beta-D-sambubioside OR 6) anthocyanidin 3-O-glucoside 6’’-O- acyltransferase -> Pelargonidin 3-(6-p-coumaroyl)glucoside &/OR Pelargonidin 3-O-(6-caffeoyl-beta-D-glucoside) -

Supplementary Materials
Supplementary Materials Table S1. Metals with stdev W1 W2 W3 W4 W5 W6 W7 W8 W9 W10 W11 W12 W13 As <LOD <LOD 19.84±0.03 <LOD <LOD <LOD <LOD <LOD <LOD <LOD <LOD <LOD <LOD Ba 32.6±0.3 33.8±0.4 26.1±0.2 123.3±0.9 79.8±0.8 79.1±0.7 83.3±0.6 239.7±0.8 58.1±0.4 77.7±0.3 32.1±0.3 31.7±0.5 122.1±0.8 Cd 0.365±0.004 1.553±0.002 0.939±0.002 1.254±0.003 0.386±0.005 1.367±0.008 0.733±0.004 0.050±0.002 0.976±0.002 1.644±0.006 <LOD 0.593±0.004 0.375±0.003 Co 3.64±0.04 2.09±0.02 0.98±0.03 4.40±0.06 2.77±0.02 1.83±0.02 2.42±0.02 2.68±0.03 7.34±0.09 7.78±0.07 2.12±0.04 1.61±0.02 2.60±0.03 Cr <LOD <LOD <LOD <LOD 4.322±0.005 3.030±0004 <LOD 6.417±0.006 15.41±0.02 14.82±0.05 11.12±0.04 9.15±0.02 3.111±0.004 Pb <LOD 56.0±0.8 <LOD 20.8±0.9 80±2 11.7±0.6 2.20±0.05 175±3 45.0±0.8 <LOD 0.830±0.007 0.538±0.006 14.9±0.3 Sb 32.8±0.5 8.59±0.09 19.1±0.2 18.±0.2 67.5±0.5 37.9±0.2 9.58±0.05 46.5±0.3 44.8±0.4 18.50±0.08 7.26±0.03 44.3±0.2 29.6±0.4 Se <LOD <LOD <LOD <LOD 1.45±0.09 0.30±0.05 0.23±0.08 25.7±0.4 2.87±0.2 2.06±0.04 2.91±0.8 2.38±0.07 2.99±0.06 Ni 4.17±0.05 46.0±0.2 0.569±0.005 30.9±0.3 15.3±0.2 5.77±0.02 35.2±0.3 13.86±0.04 50.6±0.7 17.9±0.2 9.99±0.03 0.197±0.007 5.00±0.02 Al 566±5 260.1±0.5 155.3±0.4 720±5 447±4 290.7±2 26.5±0.4 155.4±0.9 444±4 329±3 1027±10 567.7±8 236±3 Cu 97.5±0.8 469±3 36.3±0.9 10.07±0.05 <LOD <LOD <LOD <LOD 21.19±0.07 7.73±0.03 0.813±0.05 49.3±0.8 50.2±1.0 Mn 735±30 613±10 642±6 1016±6 762±3 743±5 839±8 1634±30 1732±20 1472±20 887±10 771±6 1299±10 V 4.18±0.02 1.894±0.007 0.574±0.002 <LOD 1.530±0.005 <LOD 1.948±0.006 3.179±0.004 3.235±0.007 -

Review Article Recent Applications of Mass Spectrometry in the Study of Grape and Wine Polyphenols
Hindawi Publishing Corporation ISRN Spectroscopy Volume 2013, Article ID 813563, 45 pages http://dx.doi.org/10.1155/2013/813563 Review Article Recent Applications of Mass Spectrometry in the Study of Grape and Wine Polyphenols Riccardo Flamini Consiglio per la Ricerca e la Sperimentazione in Agricoltura-Centro di Ricerca per la Viticoltura (CRA-VIT), Viale XXVIII Aprile 26, 31015 Conegliano, Italy Correspondence should be addressed to Riccardo Flamini; riccardo.�amini�entecra.it Received 24 September 2012; Accepted 12 October 2012 Academic �ditors: D.-A. Guo, �. Sta�lov, and M. Valko Copyright © 2013 Riccardo Flamini. is is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Polyphenols are the principal compounds associated with health bene�c effects of wine consumption and in general are characterized by antioxidant activities. Mass spectrometry is shown to play a very important role in the research of polyphenols in grape and wine and for the quality control of products. e so ionization of LC/MS makes these techniques suitable to study the structures of polyphenols and anthocyanins in grape extracts and to characterize polyphenolic derivatives formed in wines and correlated to the sensorial characteristics of the product. e coupling of the several MS techniques presented here is shown to be highly effective in structural characterization of the large number of low and high molecular weight polyphenols in grape and wine and also can be highly effective in the study of grape metabolomics. 1. Principal Polyphenols of Grape and Wine During winemaking the condensed (or nonhydrolyzable) tannins are transferred to the wine and contribute strongly to Polyphenols are the principal compounds associated to the sensorial characteristic of the product. -

Dadmun Cornell 0058O 11029.Pdf (1.396Mb)
EFFECT OF SUN EXPOSURE ON THE EVOLUTION AND DISTRIBUTION OF ANTHOCYANINS IN INTERSPECIFIC RED HYBRID WINEGRAPES A Thesis Presented to the Faculty of the Graduate School of Cornell University in Partial Fulfillment of the Requirements for the Degree of Master of Science by Catherine Hope Dadmun August 2020 © 2020 Catherine Hope Dadmun ABSTRACT Interspecific hybrid winegrapes are economically important in areas where environmental pressures inhibit traditional Vitis vinifera production. To clarify the effect of vine microclimate on red hybrid wine color, skin extract anthocyanins were characterized via HPLC for shaded and unshaded fruit from three economically significant cool-climate hybrid cultivars (Vitis spp): Corot noir, Maréchal Foch, and Marquette. Light exposure and berry and air temperature were monitored in Corot noir to represent generalized vine microclimate. Across all cultivars, the samples that underwent the leaf-pulling treatment (exposed samples) did not have significantly different concentrations of total anthocyanins compared to the control (shaded samples). However, certain individual anthocyanins within each cultivar demonstrated different concentrations with the exposure treatment. This work is the first step in defining the evolution of anthocyanin profiles during interspecific hybrid grape ripening to allow cool- climate wine grape growers to optimize viticultural production methods for high-quality red hybrid wines. Keywords: anthocyanin, interspecific hybrid, ripening, sunlight exposure, viticultural practice, leaf removal BIOGRAPHICAL SKETCH Catherine Dadmun joined Anna Katharine Mansfield’s group in the Department of Food Science and Technology at Cornell University in August 2018. She studies grape and wine chemistry, primarily focusing on hybrid Vitis spp. and the chemical color composition of grapes. Beyond academics, Catherine was heavily involved in the Food Science Graduate Student Organization (FSGSO), the Graduate and Professional Women’s Network (GPWomeN), and tutoring students at Beverly J.