PHYTOPLAN ® Pflanzliche Wirkstoffe Und Analytik I
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-
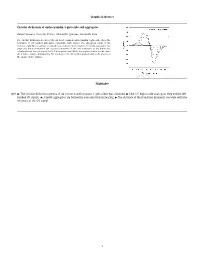
Graphical Abstract Circular Dichroism of Anthocyanidin 3-Glucoside Self
Graphical abstract Circular dichroism of anthocyanidin 3-glucoside self-aggregates Raquel Gavara, Vesselin Petrov, Alexandre Quintas, Fernando Pina * The circular dichroism spectra of the six most common anthocyanidin 3-glucoside show the formation of left handed aggregates compatible with dimers. The absorption bands of the monomer split by increasing concentration according to the formation of H and J aggregates. The angle and distance between the transition moments of the two monomers in the dimer was calculated from the splitting of the 0–0 absorption band. While the angle is similar for the series the distance changes dramatically. The intensity of the CD signal is proportional to the inverse of the square of the distance. Highlights Q10 " The circular dichroism spectra of six common anthocyanins 3-glucosides was obtained. " Like 3,5-diglucoside analogous they exhibit left- handed CD signals. " J and H aggregates are formed by concentration increasing. " The distance of the transition moments correlate with the intensity of the CD signal. 1 1 2 Circular dichroism of anthocyanidin 3-glucoside self-aggregates a a b a,⇑ 3 Q1 Raquel Gavara , Vesselin Petrov , Alexandre Quintas , Fernando Pina 4 a REQUIMTE, Departamento de Química, Faculdade de Ciências e Tecnologia, Universidade Nova de Lisboa, 2829516 Monte de Caparica, Portugal 5 b Instituto Superior de Ciencias da Saude Egas Moniz, Centro de Investigação Interdisciplinar Egas Moniz, P-2829511 Monte de Caparica, Caparica, Portugal 6 7 article info a b s t r a c t 1 9 10 Self-association constants for the flavylium cations of the six most common anthocyanidin 3-glucosides 20 11 were determined by circular dichroism (CD) and UV–Vis spectroscopy. -

Therapeutic Potential of Flavonoids in Inflammatory Bowel Disease: a Comprehensive Review
Submit a Manuscript: http://www.f6publishing.com World J Gastroenterol 2017 July 28; 23(28): 5097-5114 DOI: 10.3748/wjg.v23.i28.5097 ISSN 1007-9327 (print) ISSN 2219-2840 (online) REVIEW Therapeutic potential of flavonoids in inflammatory bowel disease: A comprehensive review Ali Salaritabar, Behrad Darvishi, Farzaneh Hadjiakhoondi, Azadeh Manayi, Antoni Sureda, Seyed Fazel Nabavi, Leo R Fitzpatrick, Seyed Mohammad Nabavi, Anupam Bishayee Ali Salaritabar, Behrad Darvishi, Department of Integrative work non-commercially, and license their derivative works on Oncology, Breast Cancer Research Center, Motamed Cancer different terms, provided the original work is properly cited and Institute, ACECR, Tehran 15179-64311, Iran the use is non-commercial. See: http://creativecommons.org/ licenses/by-nc/4.0/ Ali Salaritabar, Behrad Darvishi, Department of Recombinant Protein, Breast Cancer Research Center, Motamed Cancer Manuscript source: Invited manuscript Institute, ACECR, Tehran 15179-64311, Iran Correspondence to: Anupam Bishayee, PhD, Department Farzaneh Hadjiakhoondi, Azadeh Manayi, Medicinal Plants of Pharmaceutical Sciences, College of Pharmacy, Larkin Research Center, Faculty of Pharmacy, Tehran University of University, Miami, FL 33169, Medical Sciences, Tehran 14176-14411, Iran United States. [email protected] Telephone: +1-305-7607511 Antoni Sureda, Research Group on Community Nutrition and Oxidative Stress and CIBEROBN - Physiopathology of Obesity Received: February 16, 2017 and Nutrition, University of Balearic Islands, Palma -

Alteration of Anthocyanin Glycosylation in Cranberry Through Interspecific Hybridization
J. AMER. Soc. HORT. Sci. 130(5):711-715. 2005. Alteration of Anthocyanin Glycosylation in Cranberry Through Interspecific Hybridization Nicholi Vorsa Philip E. Marucci Center for Blueberry and Cranberry Research and Extension, Rutgers University, 125A Lake Oswego Road, Chatsworth, NJ 08019 James J. Polashock1 USDA—ARS Fruit Lab, 125A Lake Oswego Road, Chatsworth, NJ 08019 ADDITIONAL INDEX WORDS. Vaccinium macrocarpon, Vaccinium oxycoccus, antioxidant, bioavailability, flavonoid ABSTRACT. The flavonoids of american cranberry (Vaccinium macrocarpon Alt.) are documented to be beneficial for hu- man health. Among their benefits is a high antioxidant potential, with anthocyanin glycosides being the main contribu- tors. Flavonoid glucose conjugates are reported to be more bioavailable than those with other sugar conjugates. The anthocyanin glycosides of V. macrocarpon fruit are mainly galactosides and arabinosides of the aglycones, cyanidin and peonidin, with less than 8% glucosides. In contrast, the fruit anthocyanins of another cranberry species, V. oxycoccus L. were found to be largely glucosides of cyanidin and peonidin. Interspecific hybrids between these two species were intermediate to the parental species in the proportion of fruit anthocyanin glucosides. About half the progeny (1:1 segregation) in a backcross population (to V. macrocarpon) maintained the relatively high anthocyanin glucoside ratio. In this study, we demonstrate the genetic manipulation of anthocyanin glycosylation in cranberry using interspecific hybridization, resulting in dramatically increased glucose-conjugated anthocyanins. Flavonoids are considered to be secondary metabolites, which The cultivated american cranberry (V. macrocarpon) is recog- have been associated with roles in ultraviolet protection, plant nized for its brilliant red fruit due to an abundance of anthocyanins sexual reproduction, pollinator attraction, symbiotic plant—microbe in the fruit epidermal tissues. -

Current Perspectives on Medicinal and Aromatic Plants
Review Curr. Pers. MAPs, (2021); 4(1): 66-86 Current Perspectives on Medicinal and Aromatic Plants An International Journal ISSN: 2619-9645 | e-ISSN: 2667-5722 Prevention of Viral Effect and Enhancement of Immune System with the help of Herbal Plants and Himalayan Crude Drugs in SAR-COV-2 Patient: A Review Rishiram BARAL1,2 1Department of Pharmaceutical Sciences, School of Health and Allied Sciences, Faculty of Health Science, Pokhara University, Pokhara, Nepal 2Research Institute of Pharmaceutical Sciences, Department of Pharmacy, Kyungpook National University, Daegu, South Korea *Corresponding author: [email protected] Received : 07/06/2021 https://doi.org/ 10.38093/cupmap.948975 Accepted : 30/06/2021 Abstract After December 2019, Severe Acute Respiratory Syndrome (SAR-COV-2) become a life-threatening issue to the entire human society when it started to spread exponentially all over the world from Wuhan city of China. The virus directly hits the upper and lower respiratory tract of human airways and causes severe damage to the human lung, leading to multiorgan failure, hypoxemia, and dyspnea. Similarly, studies revealed that SAR-COV-2 severely hit the younger and aged population in which the immune system is seriously compromised. The cell of the immune system such as T-cells, B-cells, NK cells, etc. helps to fight against such viral antigen and resist critical viral damage. Therefore, enhancement of the immune system could also be an effective approach to prevent viral infection and even aid in the reduction of the death count. Nowadays, dietary, and herbal remedies are being integrated into the mainstream of the healthcare systems because of their multi-ingredient character, and some of them are known to render efficacy comparable to that of synthetic drug substances. -

Effects of Anthocyanins on the Ahr–CYP1A1 Signaling Pathway in Human
Toxicology Letters 221 (2013) 1–8 Contents lists available at SciVerse ScienceDirect Toxicology Letters jou rnal homepage: www.elsevier.com/locate/toxlet Effects of anthocyanins on the AhR–CYP1A1 signaling pathway in human hepatocytes and human cancer cell lines a b c d Alzbeta Kamenickova , Eva Anzenbacherova , Petr Pavek , Anatoly A. Soshilov , d e e a,∗ Michael S. Denison , Michaela Zapletalova , Pavel Anzenbacher , Zdenek Dvorak a Department of Cell Biology and Genetics, Faculty of Science, Palacky University, Slechtitelu 11, 783 71 Olomouc, Czech Republic b Institute of Medical Chemistry and Biochemistry, Faculty of Medicine and Dentistry, Palacky University, Hnevotinska 3, 775 15 Olomouc, Czech Republic c Department of Pharmacology and Toxicology, Charles University in Prague, Faculty of Pharmacy in Hradec Kralove, Heyrovskeho 1203, Hradec Kralove 50005, Czech Republic d Department of Environmental Toxicology, University of California, Meyer Hall, One Shields Avenue, Davis, CA 95616-8588, USA e Institute of Pharmacology, Faculty of Medicine and Dentistry, Palacky University, Hnevotinska 3, 775 15 Olomouc, Czech Republic h i g h l i g h t s • Food constituents may interact with drug metabolizing pathways. • AhR–CYP1A1 pathway is involved in drug metabolism and carcinogenesis. • We examined effects of 21 anthocyanins on AhR–CYP1A1 signaling. • Human hepatocytes and cell lines HepG2 and LS174T were used as the models. • Tested anthocyanins possess very low potential for food–drug interactions. a r t i c l e i n f o a b s t r a c t -

Appendix 1 – Protocol for Preventing Or Reversing Chronic Disease The
Appendix 1 – Protocol for Preventing or Reversing Chronic Disease The first author has developed a protocol over the past decade for preventing or reversing chronic disease based on the following systemic medical principle: “at the present time, removal of cause is a necessary, but not necessarily sufficient, condition for restorative treatment to be effective”[1]. The protocol methodology refines the age-old principles of both reducing harm in addition to providing treatment, and allows better identification of factors that contribute to the disease process (so that they may be eliminated if possible). These contributing factors are expansive and may include a combination of Lifestyle choices (diet, exercise, smoking), iatrogenic and biotoxin exposures, environmental/occupational exposures, and psychosocial stressors. This strategy exploits the existing literature to identify patterns of biologic response using biomarkers from various modalities of diagnostic testing to capture a much broader list of potential contributing factors. Existing inflammatory bowel diseases (IBD) Biomarker Identification to Remove Contributing Factors and Implement Treatment The initial protocol steps are diagnostic. The main output of these diagnostics will be identification of the biomarker levels and symptoms that reflect abnormalities, and the directions of change required to eliminate these abnormalities. In the present study, hundreds of general and specific biomarkers and symptoms were identified from the core IBD literature. The highest frequency (based on numbers of record appearances) biomarkers and symptoms were extracted, and are listed in Table 1 (highest frequency items first, reading down each column before proceeding to the next column). They are not necessarily consensus biomarkers. They are biomarkers whose values were altered by a treatment or contributing factor, and reported in the core IBD literature. -

An Avowal of Importance of Endangered Tree Oroxylum Indicum (Linn.) Vent
Article An avowal of importance of endangered tree Oroxylum indicum (Linn.) Vent. M Gokhale and Y K Bansal* Department of Bioscience, R.D. University, Jabalpur-482 001, Madhya Pradesh, India *Correspondent author Received 2 May 2005; Accepted 5 July 2005 Abstract A small deciduous tree Oroxylum indicum (Linn.) Vent. of family Bignoniaceae, also known as Shivnak, Sonapatha, Shyonaka or Midnight horror possesses economic as well as medicinal importance. The tree was distributed throughout the greater part of India but now it is listed amongst endangered species in many areas in the country. Its conservation is urgently required. Keywords : Shivnak, Sonapatha, Shyonaka, Midnight horror, Oroxylum indicum, Medicinal plant, Endangered tree, Conservation. 7 IPC code; Int. cl. ⎯ A61K 35/78, A01G 23/00 Leaf arrangement on rachis Introduction large (90-180 cm long) and 2-3 pinnate. are numerous, reddish purple outside and Leaflet rachis is very soft and swollen at dull or pale yellow pinkish inside; Deforestation has resulted in a the junction of the branches. Leaflets are arranged in large erect racemes. Fruit is a serious damage to biodiversity and gene in 2-4 pairs, ovate, elliptic or acuminate long woody capsule up to 1m in length, resources1, 2. Naturally grown forests have in shape and glabrous in texture. Flowers containing numerous papery, flat, winged, lost many tree species and Shivnak, light seeds. The fresh root bark is soft and Oroxylum indicum (Linn.) Vent. is juicy and creamish yellow to greyish in one of them. Shivnak grows in India, colour. The taste is sweet initially later Sri Lanka, South China, Celebes, becoming bitter. -

A Biochemical Survey of Some Mendelian Factors for Flower Colour
A BIOCHEMICAL 8UP~VEY OF SOME MENDELIAN FACTOI%S FO].~ FLOWEP~ COLOU~. BY ROSE SCOTT-MONCI~IEFF. (John Inncs Horticultural Institution, London.) (With One Text-figure.) CONTENTS. PAGE P~rb I. Introductory ].17 (a) The plastid 1)igmenl~s ] 21 (b) The a,n~hoxan~hius: i~heir backgromld, co-pigment and interaction effecbs upon flower-colour v~ri~bion 122 (c) The ani~hocyauins ] 25 (c) Col[oidM condition . 131 (f) Anthoey~nins as indic~bors 132 (g) The source of tim ~nl;hoey~nins 133 ]?ar[ II, Experimental 134 A. i~ecen~ investigations: (a) 2Prim,ula si,sensis 134- (b) Pa,l)aver Rhoeas 14.1 (c) Primuln aca.ulis 147 (d) Chc.l)ranth'ss Chci,rl 148 (e) ltosa lmlyanlha . 149 (f) Pelargonium zomdc 149 (g) Lalh,ymts odor~,l,us 150 (h) Vcrbom, hybrids 153 (i) Sl;'e2)loca~'])uG hybrids 15~ (j) T'rol)aeolu,m ,majors ] 55 ]3. B,eviews of published remflts of bhe t~u~horand o~hers.. (a) Dahlia variabilis (Lawreuce and Scol,~-Monerieff) 156 (b) A.nlb'rhinum majors (Wheklalo-Onslow, :Basseb~ a,nd ,~cobb- M.oncrieff ) 157 (c) Pharbilis nil (I-Iagiwam) . 158 (d) J/it& (Sht'itl.er it,lid Anderson) • . 159 (e) Zect d]f.ctys (~&udo, Miiner trod 8borl/lall) 159 Par~, III. The generM beh~wiour of Mendelian £acbors rot' flower colour . 160 Summary . 167 tLefermmes 168 I)AI~T I. II~TI~O])UOTOnY. Slm~C~ Onslow (1914) m~de the first sfudy of biochemica] chal~ges in- volved in flower-eolour va,riadon, our pro'ely chemical knowledge of bhe 118 A Bio&emical Su~'vey oI' Factor's fo~ • Flowe~' Colou~' anthocya.nin pigments has been considerably advanced by the work of Willstgtter, P~obinson, Karrer and their collaborators. -

Ep 3138585 A1
(19) TZZ¥_¥_T (11) EP 3 138 585 A1 (12) EUROPEAN PATENT APPLICATION (43) Date of publication: (51) Int Cl.: 08.03.2017 Bulletin 2017/10 A61L 27/20 (2006.01) A61L 27/54 (2006.01) A61L 27/52 (2006.01) (21) Application number: 16191450.2 (22) Date of filing: 13.01.2011 (84) Designated Contracting States: (72) Inventors: AL AT BE BG CH CY CZ DE DK EE ES FI FR GB • Gousse, Cecile GR HR HU IE IS IT LI LT LU LV MC MK MT NL NO 74230 Dingy Saint Clair (FR) PL PT RO RS SE SI SK SM TR • Lebreton, Pierre Designated Extension States: 74000 Annecy (FR) BA ME •Prost,Nicloas 69440 Mornant (FR) (30) Priority: 13.01.2010 US 687048 26.02.2010 US 714377 (74) Representative: Hoffmann Eitle 30.11.2010 US 956542 Patent- und Rechtsanwälte PartmbB Arabellastraße 30 (62) Document number(s) of the earlier application(s) in 81925 München (DE) accordance with Art. 76 EPC: 15178823.9 / 2 959 923 Remarks: 11709184.3 / 2 523 701 This application was filed on 29-09-2016 as a divisional application to the application mentioned (71) Applicant: Allergan Industrie, SAS under INID code 62. 74370 Pringy (FR) (54) STABLE HYDROGEL COMPOSITIONS INCLUDING ADDITIVES (57) The present specification generally relates to hydrogel compositions and methods of treating a soft tissue condition using such hydrogel compositions. EP 3 138 585 A1 Printed by Jouve, 75001 PARIS (FR) EP 3 138 585 A1 Description CROSS REFERENCE 5 [0001] This patent application is a continuation-in-part of U.S. -

Pharmacokinetics of B-Ring Unsubstituted Flavones
pharmaceutics Review Pharmacokinetics of B-Ring Unsubstituted Flavones Robert Ancuceanu 1, Mihaela Dinu 1,*, Cristina Dinu-Pirvu 2, Valentina Anu¸ta 2 and Vlad Negulescu 3 1 Department of Pharmaceutical Botany and Cell Biology, Faculty of Pharmacy, Carol Davila University of Medicine and Pharmacy, Bucharest, Romania 2 Department of Physical Chemistry and Colloidal Chemistry, Faculty of Pharmacy, Carol Davila University of Medicine and Pharmacy, 020956 Bucharest 020956, Romania 3 Department of Toxicology, Clinical Pharmacology and Psychopharmacology, Faculty of Medicine, Carol Davila University of Medicine and Pharmacy, 050474 Bucharest, Romania * Correspondence: [email protected]; Tel.: +40-21-318-0746 Received: 3 July 2019; Accepted: 23 July 2019; Published: 1 August 2019 Abstract: B-ring unsubstituted flavones (of which the most widely known are chrysin, baicalein, wogonin, and oroxylin A) are 2-phenylchromen-4-one molecules of which the B-ring is devoid of any hydroxy, methoxy, or other substituent. They may be found naturally in a number of herbal products used for therapeutic purposes, and several have been designed by researchers and obtained in the laboratory. They have generated interest in the scientific community for their potential use in a variety of pathologies, and understanding their pharmacokinetics is important for a grasp of their optimal use. Based on a comprehensive survey of the relevant literature, this paper examines their absorption (with deglycosylation as a preliminary step) and their fate in the body, from metabolism to excretion. Differences among species (inter-individual) and within the same species (intra-individual) variability have been examined based on the available data, and finally, knowledge gaps and directions of future research are discussed. -

Anthocyanin Biosynthesis 21421.Pdf
Anthocyanin Biosynthesis – https://www.kegg.jp/kegg-bin/highlight_pathway?scale=1.0&map=map00942&keyword=flavonoids Anthocyanidins (aglycones) and anthocyanins (glycosides) are common plant pigments and belong to a structural subclass of flavonoids characterized by a 2- phenylbenzopyrylium unit. They are derived along the flavonoid modification pathways and further separated into three types, pelargonidin, cyanidin, and delphinidin, due to the different number of hydroxyl groups in the phenyl group. (Flavonoid Biosynthesis) -> [1,2,3] 1) Pelargonidin -> anthocyanidin 3-O-glucosyltransferase -> Pelargonidin 3-O- glucoside -> [1,2,3,4,5,6,7] 1) anthocyanin 3-O-glucoside-6’’-O- malonyltransferase -> Pelargonidin 3-O-(6-O-malonyl-beta-D-glucoside) -> anthocyanidin 3-O-glucoside-3’’,6’’-O-dimalonyltransferase -> Pelargonidin 3-O-3’’,6’’-O-dimalonylglucoside OR 2) anthocyanidin 3-O-glucoside 2’’-O- glucosyltransferase -> Pelargonidin 3-O-sophoroside OR 3) Pelargonidin 3- O-rutinoside -> cyanidin 3-O-rutinoside 5-O-glucosyltransferase -> Pelargonidin 3-O-rutinoside 5-O-beta-D-glucoside OR 4) cyanidin 3-O- glucoside 7-O-glucosyltransferase (acyl-glucose) -> Pelargonidin 3,7-di-O- beta-D-glucoside OR 5) anthocyanidin 3-O-glucoside 2’’’-O- xylosyltransferase -> Pelargonidin 3-O-beta-D-sambubioside -> anthocyanin 3-O-sambubioside 5-O-glucosyltransferase -> Pelargonidin 5-O-beta-D- glucoside 3-O-beta-D-sambubioside OR 6) anthocyanidin 3-O-glucoside 6’’-O- acyltransferase -> Pelargonidin 3-(6-p-coumaroyl)glucoside &/OR Pelargonidin 3-O-(6-caffeoyl-beta-D-glucoside) -

Supplementary Materials
Supplementary Materials Table S1. Metals with stdev W1 W2 W3 W4 W5 W6 W7 W8 W9 W10 W11 W12 W13 As <LOD <LOD 19.84±0.03 <LOD <LOD <LOD <LOD <LOD <LOD <LOD <LOD <LOD <LOD Ba 32.6±0.3 33.8±0.4 26.1±0.2 123.3±0.9 79.8±0.8 79.1±0.7 83.3±0.6 239.7±0.8 58.1±0.4 77.7±0.3 32.1±0.3 31.7±0.5 122.1±0.8 Cd 0.365±0.004 1.553±0.002 0.939±0.002 1.254±0.003 0.386±0.005 1.367±0.008 0.733±0.004 0.050±0.002 0.976±0.002 1.644±0.006 <LOD 0.593±0.004 0.375±0.003 Co 3.64±0.04 2.09±0.02 0.98±0.03 4.40±0.06 2.77±0.02 1.83±0.02 2.42±0.02 2.68±0.03 7.34±0.09 7.78±0.07 2.12±0.04 1.61±0.02 2.60±0.03 Cr <LOD <LOD <LOD <LOD 4.322±0.005 3.030±0004 <LOD 6.417±0.006 15.41±0.02 14.82±0.05 11.12±0.04 9.15±0.02 3.111±0.004 Pb <LOD 56.0±0.8 <LOD 20.8±0.9 80±2 11.7±0.6 2.20±0.05 175±3 45.0±0.8 <LOD 0.830±0.007 0.538±0.006 14.9±0.3 Sb 32.8±0.5 8.59±0.09 19.1±0.2 18.±0.2 67.5±0.5 37.9±0.2 9.58±0.05 46.5±0.3 44.8±0.4 18.50±0.08 7.26±0.03 44.3±0.2 29.6±0.4 Se <LOD <LOD <LOD <LOD 1.45±0.09 0.30±0.05 0.23±0.08 25.7±0.4 2.87±0.2 2.06±0.04 2.91±0.8 2.38±0.07 2.99±0.06 Ni 4.17±0.05 46.0±0.2 0.569±0.005 30.9±0.3 15.3±0.2 5.77±0.02 35.2±0.3 13.86±0.04 50.6±0.7 17.9±0.2 9.99±0.03 0.197±0.007 5.00±0.02 Al 566±5 260.1±0.5 155.3±0.4 720±5 447±4 290.7±2 26.5±0.4 155.4±0.9 444±4 329±3 1027±10 567.7±8 236±3 Cu 97.5±0.8 469±3 36.3±0.9 10.07±0.05 <LOD <LOD <LOD <LOD 21.19±0.07 7.73±0.03 0.813±0.05 49.3±0.8 50.2±1.0 Mn 735±30 613±10 642±6 1016±6 762±3 743±5 839±8 1634±30 1732±20 1472±20 887±10 771±6 1299±10 V 4.18±0.02 1.894±0.007 0.574±0.002 <LOD 1.530±0.005 <LOD 1.948±0.006 3.179±0.004 3.235±0.007