An Efficient Method for High-Purity Anthocyanin Isomers Isolation From
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
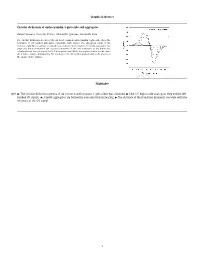
Graphical Abstract Circular Dichroism of Anthocyanidin 3-Glucoside Self
Graphical abstract Circular dichroism of anthocyanidin 3-glucoside self-aggregates Raquel Gavara, Vesselin Petrov, Alexandre Quintas, Fernando Pina * The circular dichroism spectra of the six most common anthocyanidin 3-glucoside show the formation of left handed aggregates compatible with dimers. The absorption bands of the monomer split by increasing concentration according to the formation of H and J aggregates. The angle and distance between the transition moments of the two monomers in the dimer was calculated from the splitting of the 0–0 absorption band. While the angle is similar for the series the distance changes dramatically. The intensity of the CD signal is proportional to the inverse of the square of the distance. Highlights Q10 " The circular dichroism spectra of six common anthocyanins 3-glucosides was obtained. " Like 3,5-diglucoside analogous they exhibit left- handed CD signals. " J and H aggregates are formed by concentration increasing. " The distance of the transition moments correlate with the intensity of the CD signal. 1 1 2 Circular dichroism of anthocyanidin 3-glucoside self-aggregates a a b a,⇑ 3 Q1 Raquel Gavara , Vesselin Petrov , Alexandre Quintas , Fernando Pina 4 a REQUIMTE, Departamento de Química, Faculdade de Ciências e Tecnologia, Universidade Nova de Lisboa, 2829516 Monte de Caparica, Portugal 5 b Instituto Superior de Ciencias da Saude Egas Moniz, Centro de Investigação Interdisciplinar Egas Moniz, P-2829511 Monte de Caparica, Caparica, Portugal 6 7 article info a b s t r a c t 1 9 10 Self-association constants for the flavylium cations of the six most common anthocyanidin 3-glucosides 20 11 were determined by circular dichroism (CD) and UV–Vis spectroscopy. -

Isolation of Anthocyanins by High-Speed Countercurrent
ition & F tr oo u d N f S č o c Veli kovska et al., J Nutr Food Sci 2013, 3:6 l i e a n n c r e DOI: 10.4172/2155-9600.1000243 u s o J Journal of Nutrition & Food Sciences ISSN: 2155-9600 Research Article Open Access Isolation of Anthocyanins by High-Speed Countercurrent Chromatography and Application of the Color Activity Concept to Different Varieties of Red Grape Pomace from Macedonia Sanja Kostadinović Veličkovska1*, Hamed Mirhosseini2 and Elena Bogeva3 1Faculty of Agriculture, University “Goce Delčev”, Krste Misirkov bb, 2000 Štip, Macedonia 2Department of Food Technology, Faculty of Food Science and Technology, University Putra Malaysia, Sri Serdang, 43400 UPM Serdang, Selangor, Malaysia 3Experimental laboratory of wines and wine-making, Elenov winery, Demir Kapija, Macedonia Abstract Anthocyanins of Macedonian grape pomace from three varieties “Pinot noir”, “Merlot” and “Vranec” were isolated by high speed countercurrent chromatography. After purification of the fractions by means of preparative high performance liquid chromatography the structures of isolated pigments were elucidated by electrospray ionization multiple mass spectrometry (ESI-MSn) and nuclear magnetic resonance (NMR) spectroscopy. The major anthocyanin malvidin-3-glucoside and the minor pigments delphinidin-3-glucoside, cyaniding-3-glucoside, petunidin-3-glucoside, and malvidin-3-p-coumarоyl-glucoside were isolated. The “Color activity concept” was applied and visual detection thresholds of isolated anthocyanins were determined. The results of the “color activity value” of the isolated pigments and their detection thresholds were in good agreement with the color shade of the different varieties of red grape pomace. Keywords: Red grape pomace; HSCCC; NMR; Anthocyanins; Color using NMR spectroscopy confirmed the structure of the most abundant activity concept pigments in plants [11-20]. -

Chemistry and Pharmacology of Kinkéliba (Combretum
CHEMISTRY AND PHARMACOLOGY OF KINKÉLIBA (COMBRETUM MICRANTHUM), A WEST AFRICAN MEDICINAL PLANT By CARA RENAE WELCH A Dissertation submitted to the Graduate School-New Brunswick Rutgers, The State University of New Jersey in partial fulfillment of the requirements for the degree of Doctor of Philosophy Graduate Program in Medicinal Chemistry written under the direction of Dr. James E. Simon and approved by ______________________________ ______________________________ ______________________________ ______________________________ New Brunswick, New Jersey January, 2010 ABSTRACT OF THE DISSERTATION Chemistry and Pharmacology of Kinkéliba (Combretum micranthum), a West African Medicinal Plant by CARA RENAE WELCH Dissertation Director: James E. Simon Kinkéliba (Combretum micranthum, Fam. Combretaceae) is an undomesticated shrub species of western Africa and is one of the most popular traditional bush teas of Senegal. The herbal beverage is traditionally used for weight loss, digestion, as a diuretic and mild antibiotic, and to relieve pain. The fresh leaves are used to treat malarial fever. Leaf extracts, the most biologically active plant tissue relative to stem, bark and roots, were screened for antioxidant capacity, measuring the removal of a radical by UV/VIS spectrophotometry, anti-inflammatory activity, measuring inducible nitric oxide synthase (iNOS) in RAW 264.7 macrophage cells, and glucose-lowering activity, measuring phosphoenolpyruvate carboxykinase (PEPCK) mRNA expression in an H4IIE rat hepatoma cell line. Radical oxygen scavenging activity, or antioxidant capacity, was utilized for initially directing the fractionation; highlighted subfractions and isolated compounds were subsequently tested for anti-inflammatory and glucose-lowering activities. The ethyl acetate and n-butanol fractions of the crude leaf extract were fractionated leading to the isolation and identification of a number of polyphenolic ii compounds. -

Plant Phenolics: Bioavailability As a Key Determinant of Their Potential Health-Promoting Applications
antioxidants Review Plant Phenolics: Bioavailability as a Key Determinant of Their Potential Health-Promoting Applications Patricia Cosme , Ana B. Rodríguez, Javier Espino * and María Garrido * Neuroimmunophysiology and Chrononutrition Research Group, Department of Physiology, Faculty of Science, University of Extremadura, 06006 Badajoz, Spain; [email protected] (P.C.); [email protected] (A.B.R.) * Correspondence: [email protected] (J.E.); [email protected] (M.G.); Tel.: +34-92-428-9796 (J.E. & M.G.) Received: 22 October 2020; Accepted: 7 December 2020; Published: 12 December 2020 Abstract: Phenolic compounds are secondary metabolites widely spread throughout the plant kingdom that can be categorized as flavonoids and non-flavonoids. Interest in phenolic compounds has dramatically increased during the last decade due to their biological effects and promising therapeutic applications. In this review, we discuss the importance of phenolic compounds’ bioavailability to accomplish their physiological functions, and highlight main factors affecting such parameter throughout metabolism of phenolics, from absorption to excretion. Besides, we give an updated overview of the health benefits of phenolic compounds, which are mainly linked to both their direct (e.g., free-radical scavenging ability) and indirect (e.g., by stimulating activity of antioxidant enzymes) antioxidant properties. Such antioxidant actions reportedly help them to prevent chronic and oxidative stress-related disorders such as cancer, cardiovascular and neurodegenerative diseases, among others. Last, we comment on development of cutting-edge delivery systems intended to improve bioavailability and enhance stability of phenolic compounds in the human body. Keywords: antioxidant activity; bioavailability; flavonoids; health benefits; phenolic compounds 1. Introduction Phenolic compounds are secondary metabolites widely spread throughout the plant kingdom with around 8000 different phenolic structures [1]. -

Cyanidin-3-O-Glucoside Is an Important Anthocyanin in Several Clones of Vitis Vinifera L
LOGAN ET AL., CYANIDIN-3-O-GLUCOSIDE IN PINOT NOIR FRUITS AND WINE, P.1 CYANIDIN-3-O-GLUCOSIDE IS AN IMPORTANT ANTHOCYANIN IN SEVERAL CLONES OF VITIS VINIFERA L. PINOT NOIR FRUITS AND RESULTING WINE FROM MICHIGAN AND NEW ZEALAND. Gerard A. LOGAN 1* , G. Stanley HOWELL 2, Muraleedharan G. NAIR 3 1* Corresponding Author: Gerard A. Logan [Tel.: +64 6 974 8000, Ext.: 5832; Fax.: +64 6 974 8910; E-mail.: [email protected] ]. Lecturer in Viticulture, School of Viticulture & Wine, Faculty of Science & Technology, Eastern Institute of Technology, Gloucester Street, Private Bag 1201, Taradale, Hawkes Bay, New Zealand. 2 G. Stanley Howell, Emeritus Professor, Program of Viticulture and Enology, Department of Horticulture, Michigan State University, East Lansing, Michigan 48824, USA. 3 Muraleedharan G. Nair, Professor, Bioactive Natural Products and Phytoceuticals, Department of Horticulture and National Food Safety and Toxicology Center, Michigan State University, East Lansing, Michigan 48824, USA. Abstract In the cool winegrowing regions of Michigan, USA and Canterbury, New Zealand, Vitis vinifera L. Pinot noir is an economically important red winegrape cultivar. Both regions have problems with the color of Pinot noir wines based on anthocyanin concentration. Thus, anthocyanin concentration of V. vinifera L. Pinot noir fruit was investigated using three clones and two growing locations, Canterbury, New Zealand and Michigan, USA. Wines were made from Michigan sample vines, and analyzed for anthocyanin. Utilizing HPLC (High Pressure Liquid Chromatography) techniques, the five main anthocyanins (1-5), in the fruit and wine were identified and quantified based on cyanidin-3-O-glucoside (4, C3G), and total anthocyanin concentration in grapes and wine was compared. -

Anthocyanins, Vibrant Color Pigments, and Their Role in Skin Cancer Prevention
biomedicines Review Anthocyanins, Vibrant Color Pigments, and Their Role in Skin Cancer Prevention 1,2, , 2,3, 4,5 3 Zorit, a Diaconeasa * y , Ioana S, tirbu y, Jianbo Xiao , Nicolae Leopold , Zayde Ayvaz 6 , Corina Danciu 7, Huseyin Ayvaz 8 , Andreea Stanilˇ aˇ 1,2,Madˇ alinaˇ Nistor 1,2 and Carmen Socaciu 1,2 1 Faculty of Food Science and Technology, University of Agricultural Sciences and Veterinary Medicine, 400372 Cluj-Napoca, Romania; [email protected] (A.S.); [email protected] (M.N.); [email protected] (C.S.) 2 Institute of Life Sciences, University of Agricultural Sciences and Veterinary Medicine, Calea Mănă¸stur3-5, 400372 Cluj-Napoca, Romania; [email protected] 3 Faculty of Physics, Babes, -Bolyai University, Kogalniceanu 1, 400084 Cluj-Napoca, Romania; [email protected] 4 Institute of Chinese Medical Sciences, State Key Laboratory of Quality Research in Chinese Medicine, University of Macau, Taipa, Macau 999078, China; [email protected] 5 International Research Center for Food Nutrition and Safety, Jiangsu University, Zhenjiang 212013, China 6 Faculty of Marine Science and Technology, Department of Marine Technology Engineering, Canakkale Onsekiz Mart University, 17100 Canakkale, Turkey; [email protected] 7 Victor Babes University of Medicine and Pharmacy, Department of Pharmacognosy, 2 Eftimie Murgu Sq., 300041 Timisoara, Romania; [email protected] 8 Department of Food Engineering, Engineering Faculty, Canakkale Onsekiz Mart University, 17020 Canakkale, Turkey; [email protected] * Correspondence: [email protected]; Tel.: +40-751-033-871 These authors contributed equally to this work. y Received: 31 July 2020; Accepted: 25 August 2020; Published: 9 September 2020 Abstract: Until today, numerous studies evaluated the topic of anthocyanins and various types of cancer, regarding the anthocyanins’ preventative and inhibitory effects, underlying molecular mechanisms, and such. -

Effects of Anthocyanins on the Ahr–CYP1A1 Signaling Pathway in Human
Toxicology Letters 221 (2013) 1–8 Contents lists available at SciVerse ScienceDirect Toxicology Letters jou rnal homepage: www.elsevier.com/locate/toxlet Effects of anthocyanins on the AhR–CYP1A1 signaling pathway in human hepatocytes and human cancer cell lines a b c d Alzbeta Kamenickova , Eva Anzenbacherova , Petr Pavek , Anatoly A. Soshilov , d e e a,∗ Michael S. Denison , Michaela Zapletalova , Pavel Anzenbacher , Zdenek Dvorak a Department of Cell Biology and Genetics, Faculty of Science, Palacky University, Slechtitelu 11, 783 71 Olomouc, Czech Republic b Institute of Medical Chemistry and Biochemistry, Faculty of Medicine and Dentistry, Palacky University, Hnevotinska 3, 775 15 Olomouc, Czech Republic c Department of Pharmacology and Toxicology, Charles University in Prague, Faculty of Pharmacy in Hradec Kralove, Heyrovskeho 1203, Hradec Kralove 50005, Czech Republic d Department of Environmental Toxicology, University of California, Meyer Hall, One Shields Avenue, Davis, CA 95616-8588, USA e Institute of Pharmacology, Faculty of Medicine and Dentistry, Palacky University, Hnevotinska 3, 775 15 Olomouc, Czech Republic h i g h l i g h t s • Food constituents may interact with drug metabolizing pathways. • AhR–CYP1A1 pathway is involved in drug metabolism and carcinogenesis. • We examined effects of 21 anthocyanins on AhR–CYP1A1 signaling. • Human hepatocytes and cell lines HepG2 and LS174T were used as the models. • Tested anthocyanins possess very low potential for food–drug interactions. a r t i c l e i n f o a b s t r a c t -

Naturally Occurring Anthocyanin, Structure, Functions And
iochemis t B try n & la P P h f y o s l i Journal of o a l n o r g Pervaiz et al., J Plant Biochem Physiol 2017, 5:2 u y o J DOI: 10.4172/2329-9029.1000187 ISSN: 2329-9029 Plant Biochemistry & Physiology Review Article Open Access Naturally Occurring Anthocyanin, Structure, Functions and Biosynthetic Pathway in Fruit Plants Tariq Pervaiz1,2, Jiu Songtao1, Faezeh Faghihi3, Muhammad Salman Haider1 and Jinggui Fang1* 1Key Laboratory of Genetics and Fruit Development, College of Horticulture, Nanjing Agricultural University, Nanjing 210095, PR China 2Department of Agriculture and Food Technology, Karakoram International University Gilgit, Pakistan 3Cellular and Molecular Research Center, Iran University of Medical Sciences, Tehran, Iran *Corresponding author: Jinggui Fang, Key Laboratory of Genetics and Fruit Development, College of Horticulture, Nanjing Agricultural University, Nanjing, PR China, E-mail: [email protected] Received Date: April 25, 2017; Accepted Date: April 29, 2017; Published Date: May 06, 2017 Copyright: © 2017 Pervaiz T, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Abstract Anthocyanins are naturally occurring compounds, member of the flavonoid groups of photochemical, involved in defense against the damaging effects of UV irradiation in plants and protect from many oxidants. The anthocyanins, group of pigments are relatively small and diverse flavonoid family in nature, and responsible for the attractive colors, red and purple to blue in many plants. Presence of pigments in flowers and fruits seems to provide attraction for pollination and aiding seed distribution, it also provides antiviral and antimicrobial activities, however their occurrence in the vacuoles remains ambiguous. -

8.2 Shikimic Acid Pathway
CHAPTER 8 © Jones & Bartlett Learning, LLC © Jones & Bartlett Learning, LLC NOT FORAromatic SALE OR DISTRIBUTION and NOT FOR SALE OR DISTRIBUTION Phenolic Compounds © Jones & Bartlett Learning, LLC © Jones & Bartlett Learning, LLC NOT FOR SALE OR DISTRIBUTION NOT FOR SALE OR DISTRIBUTION © Jones & Bartlett Learning, LLC © Jones & Bartlett Learning, LLC NOT FOR SALE OR DISTRIBUTION NOT FOR SALE OR DISTRIBUTION © Jones & Bartlett Learning, LLC © Jones & Bartlett Learning, LLC NOT FOR SALE OR DISTRIBUTION NOT FOR SALE OR DISTRIBUTION © Jones & Bartlett Learning, LLC © Jones & Bartlett Learning, LLC NOT FOR SALE OR DISTRIBUTION NOT FOR SALE OR DISTRIBUTION © Jones & Bartlett Learning, LLC © Jones & Bartlett Learning, LLC NOT FOR SALE OR DISTRIBUTION NOT FOR SALE OR DISTRIBUTION CHAPTER OUTLINE Overview Synthesis and Properties of Polyketides 8.1 8.5 Synthesis of Chalcones © Jones & Bartlett Learning, LLC © Jones & Bartlett Learning, LLC 8.2 Shikimic Acid Pathway Synthesis of Flavanones and Derivatives NOT FOR SALE ORPhenylalanine DISTRIBUTION and Tyrosine Synthesis NOT FOR SALESynthesis OR DISTRIBUTION and Properties of Flavones Tryptophan Synthesis Synthesis and Properties of Anthocyanidins Synthesis and Properties of Isofl avonoids Phenylpropanoid Pathway 8.3 Examples of Other Plant Polyketide Synthases Synthesis of Trans-Cinnamic Acid Synthesis and Activity of Coumarins Lignin Synthesis Polymerization© Jonesof Monolignols & Bartlett Learning, LLC © Jones & Bartlett Learning, LLC Genetic EngineeringNOT FOR of Lignin SALE OR DISTRIBUTION NOT FOR SALE OR DISTRIBUTION Natural Products Derived from the 8.4 Phenylpropanoid Pathway Natural Products from Monolignols © Jones & Bartlett Learning, LLC © Jones & Bartlett Learning, LLC NOT FOR SALE OR DISTRIBUTION NOT FOR SALE OR DISTRIBUTION © Jones & Bartlett Learning, LLC © Jones & Bartlett Learning, LLC NOT FOR SALE OR DISTRIBUTION NOT FOR SALE OR DISTRIBUTION 119 © Jones & Bartlett Learning, LLC. -

1 Title: Methods for Rapid Screening of Muscadine Grapes for Browning
1 Title: Methods for Rapid Screening of Muscadine Grapes for Browning and Pigment Profile Progress Report Grant Code 2015-03 Research Proposal Dr. Penelope Perkins-Veazie Professor, Postharvest Physiology Horticultural Sciences Dept., North Carolina State University Plants for Human Health Institute, NCRC Suite 1321, 600 Laureate Way Kannapolis, NC 28081 [email protected] Dr. Patrick Conner University of Georgia - Tifton Campus 2360 Rainwater Road Tifton, Ga 31793-5766 [email protected] Dr. John Clark University of Arkansas 316 Plant Science Bldg Fayetteville AR 72701 [email protected] Dr. James Ballington Department of Horticultural Science North Carolina State University Raleigh NC 27695 [email protected] Dr. Guoying Ma Plants for Human Health Institute, NCRC Suite 1321, 600 Laureate Way Kannapolis, NC 28081 [email protected]! 2 Objective: 1.! Develop a rapid and simplified method to detect juice browning in muscadine selections 2.! Determine pigment profiles of purple/black muscadine grape selections and seedling populations and test browning of juice from these grapes Justification and description Muscadine (Vitis rotundifolia) grapes are native to the southern U.S. These grapes are unique in that they contain pigments that are almost all diglucosides, in contrast to the monoglucoside- type pigments found in V. vinifera. These pigments, consisting of 3,5 diglucosides of malvidin, delphinidin, peonidin, petunidin, and cyanidin, can result in browning of muscadine juices and wine over time. Work done by Ballinger et al. (1974), Flora (1978), and recently by Conner and MaClean (2013) indicate that the relative amount of malvidin 3,5 diglucoside is at least part of the solution to prevent browning; total anthocyanin content needs to be above 150 mg/100g peel dry weight as well (bronze grapes contain less than 150 mg/100g). -

A Biochemical Survey of Some Mendelian Factors for Flower Colour
A BIOCHEMICAL 8UP~VEY OF SOME MENDELIAN FACTOI%S FO].~ FLOWEP~ COLOU~. BY ROSE SCOTT-MONCI~IEFF. (John Inncs Horticultural Institution, London.) (With One Text-figure.) CONTENTS. PAGE P~rb I. Introductory ].17 (a) The plastid 1)igmenl~s ] 21 (b) The a,n~hoxan~hius: i~heir backgromld, co-pigment and interaction effecbs upon flower-colour v~ri~bion 122 (c) The ani~hocyauins ] 25 (c) Col[oidM condition . 131 (f) Anthoey~nins as indic~bors 132 (g) The source of tim ~nl;hoey~nins 133 ]?ar[ II, Experimental 134 A. i~ecen~ investigations: (a) 2Prim,ula si,sensis 134- (b) Pa,l)aver Rhoeas 14.1 (c) Primuln aca.ulis 147 (d) Chc.l)ranth'ss Chci,rl 148 (e) ltosa lmlyanlha . 149 (f) Pelargonium zomdc 149 (g) Lalh,ymts odor~,l,us 150 (h) Vcrbom, hybrids 153 (i) Sl;'e2)loca~'])uG hybrids 15~ (j) T'rol)aeolu,m ,majors ] 55 ]3. B,eviews of published remflts of bhe t~u~horand o~hers.. (a) Dahlia variabilis (Lawreuce and Scol,~-Monerieff) 156 (b) A.nlb'rhinum majors (Wheklalo-Onslow, :Basseb~ a,nd ,~cobb- M.oncrieff ) 157 (c) Pharbilis nil (I-Iagiwam) . 158 (d) J/it& (Sht'itl.er it,lid Anderson) • . 159 (e) Zect d]f.ctys (~&udo, Miiner trod 8borl/lall) 159 Par~, III. The generM beh~wiour of Mendelian £acbors rot' flower colour . 160 Summary . 167 tLefermmes 168 I)AI~T I. II~TI~O])UOTOnY. Slm~C~ Onslow (1914) m~de the first sfudy of biochemica] chal~ges in- volved in flower-eolour va,riadon, our pro'ely chemical knowledge of bhe 118 A Bio&emical Su~'vey oI' Factor's fo~ • Flowe~' Colou~' anthocya.nin pigments has been considerably advanced by the work of Willstgtter, P~obinson, Karrer and their collaborators. -

Effects of Ph on the Stability of Cyanidin and Cyanidin 3-O-Β-Glucopyranoside in Aqueous Solution
Effects of pH on the stability of cyanidin and cyanidin 3-O-β-glucopyranoside in aqueous solution Violeta P. Rakić1, Mihaela A. Skrt2, Milena N. Miljković3, Danijela A. Kostić3, Dušan T. Sokolović4, Nataša E. Poklar Ulrih2,5 1College of Agriculture and Food Technology, Prokuplje, Serbia 2Department of Food Science and Technology, Biotechnical Faculty, University of Ljubljana, Ljubljana, Slovenia 3Department of Chemistry, Faculty of Science and Mathematics, University of Niš, Niš, Serbia 4Faculty of Medicine, University of Niš, Niš, Serbia 5Centre of Excellence for Integrated Approaches in Chemistry and Biology of Proteins (CipKeBiP), Ljubljana, Slovenia Abstract The colour variation, colour intensity and stability at various pH values (2.0, 4.0, 7.0 and SCIENTIFIC PAPER 9.0) of cyanidin 3-O-β-glucopyranoside (Cy3Glc) and its aglycone cyanidin were inves- tigated during a period of 8 hours storage at 25 °C. Our data showed that pH of aqueous UDC 547.973:667.777:543.42:544 solution had impact on spectroscopic profile of cyanidin and Cy3Glc. Beginning with the most acidic solutions, increasing the pH induce bathochromic shifts of absorbance maxi- mum in the visible range for all examined pH values (with the exception pH 4.0 for cya- Hem. Ind. 69 (5) 511–522 (2015) nidin), while the presence of the 3-glucosidic substitution induce hypsochromic shift. Com- pared to cyanidin, Cy3Glc has higher colour intensity and higher stability in the whole pH doi: 10.2298/HEMIND140711072R range, except at pH 7.0. The 3-glucosidic substitution influences on the colour intensity of Cy3Glc in the alkaline region. After 8-hour incubation of Cy3Glc and cyanidin at pH 2.0 and 25 °C, 99% of Cy3Glc and only 27% of cyanidin remained unchanged.