Bbm:978-3-540-73733-9/1.Pdf
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Wo 2007/133479 A2
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (43) International Publication Date (10) International Publication Number 22 November 2007 (22.11.2007) PCT WO 2007/133479 A2 (51) International Patent Classification: Not classified (74) Agent: GODLEWSKI, Richard, J.; P.O. Box 2269, Bloomington, IN 47402-2269 (US). (21) International Application Number: (81) Designated States (unless otherwise indicated, for every PCT/US2007/0 10828 kind of national protection available): AE, AG, AL, AM, AT,AU, AZ, BA, BB, BG, BH, BR, BW, BY, BZ, CA, CH, (22) International Filing Date: 4 May 2007 (04.05.2007) CN, CO, CR, CU, CZ, DE, DK, DM, DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, HN, HR, HU, ID, IL, IN, (25) Filing Language: English IS, JP, KE, KG, KM, KN, KP, KR, KZ, LA, LC, LK, LR, LS, LT, LU, LY,MA, MD, ME, MG, MK, MN, MW, MX, (26) Publication Language: English MY, MZ, NA, NG, NI, NO, NZ, OM, PG, PH, PL, PT, RO, RS, RU, SC, SD, SE, SG, SK, SL, SM, SV, SY, TJ, TM, (30) Priority Data: TN, TR, TT, TZ, UA, UG, US, UZ, VC, VN, ZA, ZM, ZW 60/799,608 10 May 2006 (10.05.2006) US (84) Designated States (unless otherwise indicated, for every kind of regional protection available): ARIPO (BW, GH, (71) Applicants (for all designated States except US): COOK GM, KE, LS, MW, MZ, NA, SD, SL, SZ, TZ, UG, ZM, INCORPORATED [US/US]; 750 North Daniel's Way, ZW), Eurasian (AM, AZ, BY, KG, KZ, MD, RU, TJ, TM), P.O. -
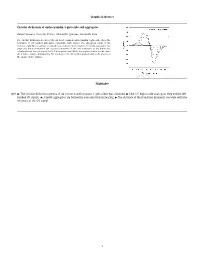
Graphical Abstract Circular Dichroism of Anthocyanidin 3-Glucoside Self
Graphical abstract Circular dichroism of anthocyanidin 3-glucoside self-aggregates Raquel Gavara, Vesselin Petrov, Alexandre Quintas, Fernando Pina * The circular dichroism spectra of the six most common anthocyanidin 3-glucoside show the formation of left handed aggregates compatible with dimers. The absorption bands of the monomer split by increasing concentration according to the formation of H and J aggregates. The angle and distance between the transition moments of the two monomers in the dimer was calculated from the splitting of the 0–0 absorption band. While the angle is similar for the series the distance changes dramatically. The intensity of the CD signal is proportional to the inverse of the square of the distance. Highlights Q10 " The circular dichroism spectra of six common anthocyanins 3-glucosides was obtained. " Like 3,5-diglucoside analogous they exhibit left- handed CD signals. " J and H aggregates are formed by concentration increasing. " The distance of the transition moments correlate with the intensity of the CD signal. 1 1 2 Circular dichroism of anthocyanidin 3-glucoside self-aggregates a a b a,⇑ 3 Q1 Raquel Gavara , Vesselin Petrov , Alexandre Quintas , Fernando Pina 4 a REQUIMTE, Departamento de Química, Faculdade de Ciências e Tecnologia, Universidade Nova de Lisboa, 2829516 Monte de Caparica, Portugal 5 b Instituto Superior de Ciencias da Saude Egas Moniz, Centro de Investigação Interdisciplinar Egas Moniz, P-2829511 Monte de Caparica, Caparica, Portugal 6 7 article info a b s t r a c t 1 9 10 Self-association constants for the flavylium cations of the six most common anthocyanidin 3-glucosides 20 11 were determined by circular dichroism (CD) and UV–Vis spectroscopy. -

The Use of Plants in the Traditional Management of Diabetes in Nigeria: Pharmacological and Toxicological Considerations
Journal of Ethnopharmacology 155 (2014) 857–924 Contents lists available at ScienceDirect Journal of Ethnopharmacology journal homepage: www.elsevier.com/locate/jep Review The use of plants in the traditional management of diabetes in Nigeria: Pharmacological and toxicological considerations Udoamaka F. Ezuruike n, Jose M. Prieto 1 Center for Pharmacognosy and Phytotherapy, Department of Pharmaceutical and Biological Chemistry, School of Pharmacy, University College London, 29-39 Brunswick Square, WC1N 1AX London, United Kingdom article info abstract Article history: Ethnopharmacological relevance: The prevalence of diabetes is on a steady increase worldwide and it is Received 15 November 2013 now identified as one of the main threats to human health in the 21st century. In Nigeria, the use of Received in revised form herbal medicine alone or alongside prescription drugs for its management is quite common. We hereby 26 May 2014 carry out a review of medicinal plants traditionally used for diabetes management in Nigeria. Based on Accepted 26 May 2014 the available evidence on the species' pharmacology and safety, we highlight ways in which their Available online 12 June 2014 therapeutic potential can be properly harnessed for possible integration into the country's healthcare Keywords: system. Diabetes Materials and methods: Ethnobotanical information was obtained from a literature search of electronic Nigeria databases such as Google Scholar, Pubmed and Scopus up to 2013 for publications on medicinal plants Ethnopharmacology used in diabetes management, in which the place of use and/or sample collection was identified as Herb–drug interactions Nigeria. ‘Diabetes’ and ‘Nigeria’ were used as keywords for the primary searches; and then ‘Plant name – WHO Traditional Medicine Strategy accepted or synonyms’, ‘Constituents’, ‘Drug interaction’ and/or ‘Toxicity’ for the secondary searches. -

The Cyanogenic Polymorphism in Trifolium Repens L
Heredity66 (1991) 105—115 Received 16 May 1990 Genetical Society of Great Britain The cyanogenic polymorphism in Trifolium repens L. (white clover) M. A. HUGHES Department of Biochemistry and Genetics, The Medical School, The University, Newcastle upon Tyne NE2 4HH Thecyanogenic polymorphism in white clover is controlled by alleles of two independently segregating loci. Biochemical studies have shown that non-functional alleles of the Ac locus, which controls the level of cyanoglucoside produced in leaf tissue, result in the loss of several steps in the biosynthetic pathway. Alleles of the Li locus control the synthesis of the hydrolytic enzyme, linamarase, which is responsible for HCN release following tissue damage. Studies on the selective forces and the distribution of the cyanogenic morphs of white clover are discussed in relation to the quantitative variation in cyanogenesis revealed by biochemical studies. Molecular studies reveal considerable restriction fragment length polymorphism for linamarase homologous genes. Keywords:cyanogenesis,polymorphism, Trifolium repen, white clover. genetics to plant taxomony. This review discusses the Introduction extensive and diverse ecological genetic studies in rela- Theterm cyanogenesis describes the release of hydro- tion to the more recent biochemical and molecular cyanic acid (HCN), which occurs when the tissues of studies of cyanogenesis in white clover. some plant species are damaged. The first report of cyanogenesis in Trifolium repens (white clover) was by Mirande (1912) and this was shortly followed by a Biochemistry paper which demonstrated that the species was poly- Theproduction of HCN by higher plants depends morphic for the character, with both cyanogenic and upon the co-occurrence of a cyanogenic glycoside and acyanogenic plants occurring in the same population catabolic enzymes. -

A Combined Experimental and Computational Study of Chrysanthemin As a Pigment for Dye-Sensitized Solar Cells
molecules Article A Combined Experimental and Computational Study of Chrysanthemin as a Pigment for Dye-Sensitized Solar Cells Atoumane Ndiaye 1,2 , Alle Dioum 1, Corneliu I. Oprea 2 , Anca Dumbrava 3,* , Jeanina Lungu 2, Adrian Georgescu 2, Florin Moscalu 2, Mihai A. Gîr¸tu 2,* , Aboubaker Chedikh Beye 1 and Issakha Youm 1,* 1 Department of Physics, Cheikh Anta Diop University of Dakar, 5005 Dakar-Fann, Senegal; [email protected] (A.N.); [email protected] (A.D.); [email protected] (A.C.B.) 2 Department of Physics, Ovidius University of Constanta, 900527 Constanta, Romania; [email protected] (C.I.O.); [email protected] (J.L.); [email protected] (A.G.); fl[email protected] (F.M.) 3 Department of Chemistry and Chemical Engineering, Ovidius University of Constanta, 900527 Constanta, Romania * Correspondence: [email protected] (A.D.); [email protected] (M.A.G.); [email protected] (I.Y.) Abstract: The theoretical study of chrysanthemin (cyanidin 3-glucoside) as a pigment for TiO2-based dye-sensitized solar cells (DSSCs) was performed with the GAUSSSIAN 09 simulation. The electronic spectra of neutral and anionic chrysanthemin molecules were calculated by density functional theory with B3LYP functional and DGDZVP basis set. A better energy level alignment was found for partially deprotonated molecules of chrysanthemin, with the excited photoelectron having enough energy in order to be transferred to the conduction band of TiO2 semiconductor in DSSCs. In addition, we used the raw aqueous extracts of roselle (Hibiscus sabdariffa) calyces as the source of chrysanthemin Citation: Ndiaye, A.; Dioum, A.; and the extracts with various pH values were tested in DSSCs. -

Chronic Cassava Toxicity
4/63 7L ARCHIV NESTEL C-010e 26528 II Chronic Cassava Toxicity Proceedings of an interdisciplinary workshop London, England, 29-30 January 1973 Editors: Barry Nestel and Reginald Maclntyre INTERNATIONAL CENTRE DE RECHERCHES DEVELOPMENT POUR LE DEVELOPPEMENT RESEARCH CENTRE INTERNATIONAL Oawa, Canada 1973 IDRC-OlOe CHRONIC CASSAVA TOXICITY Proceedings of an interdisciplinary workshop London, England, 29-30 January 1973 Editors: BARRY NESTEL AND REGINALD MACINTYRE 008817 UDC: 615.9:547.49 633.68 © 1973 International Development Research Centre Head Office: Box 8500, Ottawa, Canada. K1G 3H9 Microfiche Edition S 1 Contents Foreword Barry Nestel 5-7 Workshop Participants 8-10 Current utilization and future potential for cassava Barry Nestel 11-26 Cassava as food: toxicity and technology D. G. Coursey 27-36 Cyanide toxicity in relation to the cassava research program of CIAT in Colombia James H. Cock 37-40 Cyanide toxicity and cassava research at the International Institute of Tropical Agriculture, Ibadan, Nigeria Sidki Sadik and Sang Ki Hahn 41-42 The cyanogenic character of cassava (Manihor esculenta) G. H. de Bruijn 43-48 The genetics of cyanogenesis Monica A. Hughes 49-54 Cyanogenic glycosides: their occurrence, biosynthesis, and function Eric E. Conn 55-63 Physiological and genetic aspects of cyanogenesis in cassava and other plants G. W. Butler, P. F. Reay, and B. A. Tapper 65-71 Biosynthesis of cyanogenic glucosides in cassava (Manihot spp.) Frederick Nartey 73-87 Assay methods for hydrocyanic acid in plant tissues and their application in studies of cyanogenic glycosides in Manihot esculenta A. Zitnak 89-96 The mode of cyanide detoxication 0. -

Dr. Duke's Phytochemical and Ethnobotanical Databases List of Chemicals for Tuberculosis
Dr. Duke's Phytochemical and Ethnobotanical Databases List of Chemicals for Tuberculosis Chemical Activity Count (+)-3-HYDROXY-9-METHOXYPTEROCARPAN 1 (+)-8HYDROXYCALAMENENE 1 (+)-ALLOMATRINE 1 (+)-ALPHA-VINIFERIN 3 (+)-AROMOLINE 1 (+)-CASSYTHICINE 1 (+)-CATECHIN 10 (+)-CATECHIN-7-O-GALLATE 1 (+)-CATECHOL 1 (+)-CEPHARANTHINE 1 (+)-CYANIDANOL-3 1 (+)-EPIPINORESINOL 1 (+)-EUDESMA-4(14),7(11)-DIENE-3-ONE 1 (+)-GALBACIN 2 (+)-GALLOCATECHIN 3 (+)-HERNANDEZINE 1 (+)-ISOCORYDINE 2 (+)-PSEUDOEPHEDRINE 1 (+)-SYRINGARESINOL 1 (+)-SYRINGARESINOL-DI-O-BETA-D-GLUCOSIDE 2 (+)-T-CADINOL 1 (+)-VESTITONE 1 (-)-16,17-DIHYDROXY-16BETA-KAURAN-19-OIC 1 (-)-3-HYDROXY-9-METHOXYPTEROCARPAN 1 (-)-ACANTHOCARPAN 1 (-)-ALPHA-BISABOLOL 2 (-)-ALPHA-HYDRASTINE 1 Chemical Activity Count (-)-APIOCARPIN 1 (-)-ARGEMONINE 1 (-)-BETONICINE 1 (-)-BISPARTHENOLIDINE 1 (-)-BORNYL-CAFFEATE 2 (-)-BORNYL-FERULATE 2 (-)-BORNYL-P-COUMARATE 2 (-)-CANESCACARPIN 1 (-)-CENTROLOBINE 1 (-)-CLANDESTACARPIN 1 (-)-CRISTACARPIN 1 (-)-DEMETHYLMEDICARPIN 1 (-)-DICENTRINE 1 (-)-DOLICHIN-A 1 (-)-DOLICHIN-B 1 (-)-EPIAFZELECHIN 2 (-)-EPICATECHIN 6 (-)-EPICATECHIN-3-O-GALLATE 2 (-)-EPICATECHIN-GALLATE 1 (-)-EPIGALLOCATECHIN 4 (-)-EPIGALLOCATECHIN-3-O-GALLATE 1 (-)-EPIGALLOCATECHIN-GALLATE 9 (-)-EUDESMIN 1 (-)-GLYCEOCARPIN 1 (-)-GLYCEOFURAN 1 (-)-GLYCEOLLIN-I 1 (-)-GLYCEOLLIN-II 1 2 Chemical Activity Count (-)-GLYCEOLLIN-III 1 (-)-GLYCEOLLIN-IV 1 (-)-GLYCINOL 1 (-)-HYDROXYJASMONIC-ACID 1 (-)-ISOSATIVAN 1 (-)-JASMONIC-ACID 1 (-)-KAUR-16-EN-19-OIC-ACID 1 (-)-MEDICARPIN 1 (-)-VESTITOL 1 (-)-VESTITONE 1 -

Ultrasound-Assisted Extraction Optimization Using
a ISSN 0101-2061 (Print) Food Science and Technology ISSN 1678-457X (Online) DOI: https://doi.org/10.1590/fst.13421 Berberis crataegina DC. as a novel natural food colorant source: ultrasound-assisted extraction optimization using response surface methodology and thermal stability studies Mehmet DEMIRCI1,2 , Merve TOMAS1 , Zeynep Hazal TEKIN-ÇAKMAK2 , Salih KARASU2* Abstract This study aimed to investigate the potential use of anthocyanin of Berberis crataegina DC. as a natural food coloring agent in the food industry. For this aim, the ultrasound-assisted extraction (UAE) method was performed to extract anthocyanin of Berberis crataegina DC. The effect of ultrasound power 1(X : 20-100%), extraction temperature (X2: 20-60 °C), and time (X3: 10-20 min) on TPC and TAC of Berberis crataegina DC. extracts were examined and optimized by applying the Box–Behnken experimental design (BBD) with the response surface methodology (RSM). The influence of three independent variables and their combinatorial interactions on TPC and TAC were investigated by the quadratic models (R2: 0.9638&0.9892 and adj R2:0.9171&0.9654, respectively). The optimum conditions were determined as the amplitude level of 98%, the temperature of 57.41 °C, and extraction time of 13.86 min. The main anthocyanin compounds were identified, namely, Delphinidin-3-O- galactoside, Cyanidin-3-O-glucoside, Cyanidin-3-O-rutinoside, Petunidin-3-O-glucoside, Pelargonidin-3-O-glucoside, and Peonidin-3-O-glucoside. The anthocyanin degradation showed first-order kinetic, degradation rate constant (k), the half-life values (t1/2), and loss (%) were significantly affected by different temperatures (P < 0.05). -

3-Deoxyanthocyanins : Chemical Synthesis, Structural Transformations, Affinity for Metal Ions and Serum Albumin, Antioxidant Activity
ACADÉMIE D’AIX-MARSEILLE UNIVERSITÉ D’AVIGNON Ecole Doctorale 536 Agrosciences & Sciences THESE présentée pour l’obtention du Diplôme de Doctorat Spécialité: chimie par Sheiraz AL BITTAR le 17 juin 2016 3-Deoxyanthocyanins : Chemical synthesis, structural transformations, affinity for metal ions and serum albumin, antioxidant activity Composition du jury: Victor DE FREITAS Professeur Rapporteur Faculté des Sciences - Université de Porto Cédric SAUCIER Professeur Rapporteur Faculté de Pharmacie - Université de Montpellier I Hélène FULCRAND Directrice de Recherche à l’INRA Examinatrice Montpellier - SupAgro Olivier DANGLES Professeur Directeur de thèse UFR STS - Université d’Avignon Nathalie MORA- Maître de Conférences Co-Encadrante SOUMILLE UFR STS - Université d’Avignon A Alma & Jana… 2 Remerciements Difficile d’être exhaustive dans ces remerciements tant les rencontres, échanges et soutiens ont été nombreux durant ces cinq années. Tout d’abord, je tiens à remercier l’université d’Avignon pour m’accueillir dans ces locaux et de m’offrir le nécessaire pour acomplir ce travail. Je remercie également l’université Al-Baath en Syrie pour la bourse d’étude qui m’a permis de venir en France et Campus Farnce pour l’accueil et la direction en France. Toute ma gratitude va aux membres du jury Victor DE FREITAS, Cédric SAUCIER et Hélène FULCRAND d’avoir accepté d’évaluer ma thèse. Je remercie encore une fois Hélène FULCRAND tant que membre de mon comité de thèse, pour les discussions constructives et ses conseils pendant ma thèse. Je tiens à remercier infiniment mon directeur de thèse Olivier DANGLES. Merci d’accepter de m’accueillir dans votre équipe sans me connaitre il y a 6 ans. -

EEE M W 24B 24A 27B 27A N Patent Application Publication Dec
US 2009031 1494A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2009/0311494 A1 YAMASHTA et al. (43) Pub. Date: Dec. 17, 2009 (54) RELIEF PRINTING PLATE PRECURSOR FOR (30) Foreign Application Priority Data LASER ENGRAVING, RELIEF PRINTING PLATE, AND PROCESS FOR PRODUCING Jun. 17, 2008 (JP) ................................. 2008-157907 RELEF PRINTING PLATE Feb. 10, 2009 (JP) ................................. 2009-028816 (75) Inventors: Masako YAMASHITA, Publication Classification Shizuoka-ken (JP); Atsushi (51) Int. Cl. SUGASAKI, Shizuoka-ken (JP) B32B 3/00 (2006.01) Correspondence Address: GO3F 7/20 (2006.01) Moss & Burke, PLLC GO3F 7/004 (2006.01) 401 Holland Lane, Suite 407 Alexandria, VA 22314 (US) (52) U.S. Cl. .................... 428/195.1: 430/306: 430/286.1 (73) Assignee: FUJIFILM CORPORATION, (57) ABSTRACT Tokyo (JP) A relief printing plate precursor for laser engraving, including (21) Appl. No.: 12/476,260 a relief forming layer containing (A) a polymerizable com pound having an ethylenic unsaturated bond. (B) a binder (22) Filed: Jun. 2, 2009 polymer, and (C) a compound having deodorizing ability. 11 50 FA - 42 SUB SCANNING DIRECTION -10 - 228 7.s 55 21B EEE m w 24B 24A 27B 27A N Patent Application Publication Dec. 17, 2009 US 2009/0311494 A1 F.G. 1 FA SCANNING DIRECTION 7OA a. CSy ra & 5A - 27WSNS AD 23Ar S3EEASEE21 E-25sagaa EEEEEEEEEEEEEEEEEEEEEEEEE-22s awslighlights fskillsw. 21B 2 TTT "TT". US 2009/031 1494 A1 Dec. 17, 2009 RELEF PRINTING PLATE PRECURSORFOR mask to develop and remove an uncured area, and there is LASER ENGRAVING, RELIEF PRINTING room for improvement since development treatment is nec PLATE, AND PROCESS FOR PRODUCING essary. -

Hydroxylase in Sorghum Mesocotyls Synthesizing 3-Deoxyanthocyanidin Phytoalexins
University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln Agronomy & Horticulture -- Faculty Publications Agronomy and Horticulture Department 2004 Expression of a putative flavonoid 3'-hydroxylase in sorghum mesocotyls synthesizing 3-deoxyanthocyanidin phytoalexins Jayanand Boddu Pennsylvania State University Catherine Svabek Pennsylvania State University Rajandeep Sekhon Pennsylvania State University Amanda Gevens Michigan State University Ralph L. Nicholson Purdue University See next page for additional authors Follow this and additional works at: https://digitalcommons.unl.edu/agronomyfacpub Part of the Agricultural Science Commons, Agriculture Commons, Agronomy and Crop Sciences Commons, Botany Commons, Horticulture Commons, Other Plant Sciences Commons, and the Plant Biology Commons Boddu, Jayanand; Svabek, Catherine; Sekhon, Rajandeep; Gevens, Amanda; Nicholson, Ralph L.; Jones, A. Daniel; Pedersen, Jeffrey F.; Gustine, David L.; and Chopra, Surinder, "Expression of a putative flavonoid 3'- hydroxylase in sorghum mesocotyls synthesizing 3-deoxyanthocyanidin phytoalexins" (2004). Agronomy & Horticulture -- Faculty Publications. 939. https://digitalcommons.unl.edu/agronomyfacpub/939 This Article is brought to you for free and open access by the Agronomy and Horticulture Department at DigitalCommons@University of Nebraska - Lincoln. It has been accepted for inclusion in Agronomy & Horticulture -- Faculty Publications by an authorized administrator of DigitalCommons@University of Nebraska - Lincoln. Authors Jayanand -

Research Article Simultaneous Extraction Optimization And
Hindawi Publishing Corporation ISRN Biotechnology Volume 2013, Article ID 450948, 10 pages http://dx.doi.org/10.5402/2013/450948 Research Article Simultaneous Extraction Optimization and Analysis of Flavonoids from the Flowers of Tabernaemontana heyneana by High Performance Liquid Chromatography Coupled to Diode Array Detector and Electron Spray Ionization/Mass Spectrometry Thiyagarajan Sathishkumar,1 Ramakrishnan Baskar,1 Mohan Aravind,1 Suryanarayanan Tilak,1 Sri Deepthi,1 and Vellalore Maruthachalam Bharathikumar2 1 Department of Biotechnology, Kumaraguru College of Technology, Coimbatore 641049, India 2 Department of Biochemistry, University of Saskatchewan, Saskatoon, SK, Canada S7N 5E5 Correspondence should be addressed to iyagarajan Sathishkumar; [email protected] Received 24 June 2012; Accepted 9 August 2012 Academic Editors: Y. H. Cheong, H. Kakeshita, W. A. Kues, and D. Pant Copyright © 2013 iyagarajan Sathishkumar et al. is is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Flavonoids are exploited as antioxidants, antimicrobial, antithrombogenic, antiviral, and antihypercholesterolemic agents. Normally, conventional extraction techniques like soxhlet or shake �ask methods provide low yield of �avonoids with structural loss, and thereby, these techniques may be considered as inefficient. In this regard, an attempt was made to optimize the �avonoid extraction using orthogonal design of experiment and subsequent structural elucidation by high-performance liquid chromatography-diode array detector-electron spray ionization/mass spectrometry (HPLC-DAD-ESI/MS) techniques. e shake �ask method of �avonoid extraction was observed to provide a yield of (mg/g tissue). With the two different solvents, namely, ethanol and ethyl acetate, tried for the extraction optimization of �avonoid, ethanol (80.1 mg/g tissue) has been proved better than ethyl acetate (20.5 mg/g tissue).