The Role of Phytotoxic and Antimicrobial Compounds of Euphorbia Gummifera in the Cause and Maintenance of the Fairy Circles of Namibia
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

TRP Mediation
molecules Review Remedia Sternutatoria over the Centuries: TRP Mediation Lujain Aloum 1 , Eman Alefishat 1,2,3 , Janah Shaya 4 and Georg A. Petroianu 1,* 1 Department of Pharmacology, College of Medicine and Health Sciences, Khalifa University of Science and Technology, Abu Dhabi 127788, United Arab Emirates; [email protected] (L.A.); Eman.alefi[email protected] (E.A.) 2 Center for Biotechnology, Khalifa University of Science and Technology, Abu Dhabi 127788, United Arab Emirates 3 Department of Biopharmaceutics and Clinical Pharmacy, Faculty of Pharmacy, The University of Jordan, Amman 11941, Jordan 4 Pre-Medicine Bridge Program, College of Medicine and Health Sciences, Khalifa University of Science and Technology, Abu Dhabi 127788, United Arab Emirates; [email protected] * Correspondence: [email protected]; Tel.: +971-50-413-4525 Abstract: Sneezing (sternutatio) is a poorly understood polysynaptic physiologic reflex phenomenon. Sneezing has exerted a strange fascination on humans throughout history, and induced sneezing was widely used by physicians for therapeutic purposes, on the assumption that sneezing eliminates noxious factors from the body, mainly from the head. The present contribution examines the various mixtures used for inducing sneezes (remedia sternutatoria) over the centuries. The majority of the constituents of the sneeze-inducing remedies are modulators of transient receptor potential (TRP) channels. The TRP channel superfamily consists of large heterogeneous groups of channels that play numerous physiological roles such as thermosensation, chemosensation, osmosensation and mechanosensation. Sneezing is associated with the activation of the wasabi receptor, (TRPA1), typical ligand is allyl isothiocyanate and the hot chili pepper receptor, (TRPV1), typical agonist is capsaicin, in the vagal sensory nerve terminals, activated by noxious stimulants. -

The Cyanogenic Polymorphism in Trifolium Repens L
Heredity66 (1991) 105—115 Received 16 May 1990 Genetical Society of Great Britain The cyanogenic polymorphism in Trifolium repens L. (white clover) M. A. HUGHES Department of Biochemistry and Genetics, The Medical School, The University, Newcastle upon Tyne NE2 4HH Thecyanogenic polymorphism in white clover is controlled by alleles of two independently segregating loci. Biochemical studies have shown that non-functional alleles of the Ac locus, which controls the level of cyanoglucoside produced in leaf tissue, result in the loss of several steps in the biosynthetic pathway. Alleles of the Li locus control the synthesis of the hydrolytic enzyme, linamarase, which is responsible for HCN release following tissue damage. Studies on the selective forces and the distribution of the cyanogenic morphs of white clover are discussed in relation to the quantitative variation in cyanogenesis revealed by biochemical studies. Molecular studies reveal considerable restriction fragment length polymorphism for linamarase homologous genes. Keywords:cyanogenesis,polymorphism, Trifolium repen, white clover. genetics to plant taxomony. This review discusses the Introduction extensive and diverse ecological genetic studies in rela- Theterm cyanogenesis describes the release of hydro- tion to the more recent biochemical and molecular cyanic acid (HCN), which occurs when the tissues of studies of cyanogenesis in white clover. some plant species are damaged. The first report of cyanogenesis in Trifolium repens (white clover) was by Mirande (1912) and this was shortly followed by a Biochemistry paper which demonstrated that the species was poly- Theproduction of HCN by higher plants depends morphic for the character, with both cyanogenic and upon the co-occurrence of a cyanogenic glycoside and acyanogenic plants occurring in the same population catabolic enzymes. -

Chronic Cassava Toxicity
4/63 7L ARCHIV NESTEL C-010e 26528 II Chronic Cassava Toxicity Proceedings of an interdisciplinary workshop London, England, 29-30 January 1973 Editors: Barry Nestel and Reginald Maclntyre INTERNATIONAL CENTRE DE RECHERCHES DEVELOPMENT POUR LE DEVELOPPEMENT RESEARCH CENTRE INTERNATIONAL Oawa, Canada 1973 IDRC-OlOe CHRONIC CASSAVA TOXICITY Proceedings of an interdisciplinary workshop London, England, 29-30 January 1973 Editors: BARRY NESTEL AND REGINALD MACINTYRE 008817 UDC: 615.9:547.49 633.68 © 1973 International Development Research Centre Head Office: Box 8500, Ottawa, Canada. K1G 3H9 Microfiche Edition S 1 Contents Foreword Barry Nestel 5-7 Workshop Participants 8-10 Current utilization and future potential for cassava Barry Nestel 11-26 Cassava as food: toxicity and technology D. G. Coursey 27-36 Cyanide toxicity in relation to the cassava research program of CIAT in Colombia James H. Cock 37-40 Cyanide toxicity and cassava research at the International Institute of Tropical Agriculture, Ibadan, Nigeria Sidki Sadik and Sang Ki Hahn 41-42 The cyanogenic character of cassava (Manihor esculenta) G. H. de Bruijn 43-48 The genetics of cyanogenesis Monica A. Hughes 49-54 Cyanogenic glycosides: their occurrence, biosynthesis, and function Eric E. Conn 55-63 Physiological and genetic aspects of cyanogenesis in cassava and other plants G. W. Butler, P. F. Reay, and B. A. Tapper 65-71 Biosynthesis of cyanogenic glucosides in cassava (Manihot spp.) Frederick Nartey 73-87 Assay methods for hydrocyanic acid in plant tissues and their application in studies of cyanogenic glycosides in Manihot esculenta A. Zitnak 89-96 The mode of cyanide detoxication 0. -
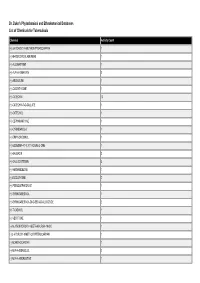
Dr. Duke's Phytochemical and Ethnobotanical Databases List of Chemicals for Tuberculosis
Dr. Duke's Phytochemical and Ethnobotanical Databases List of Chemicals for Tuberculosis Chemical Activity Count (+)-3-HYDROXY-9-METHOXYPTEROCARPAN 1 (+)-8HYDROXYCALAMENENE 1 (+)-ALLOMATRINE 1 (+)-ALPHA-VINIFERIN 3 (+)-AROMOLINE 1 (+)-CASSYTHICINE 1 (+)-CATECHIN 10 (+)-CATECHIN-7-O-GALLATE 1 (+)-CATECHOL 1 (+)-CEPHARANTHINE 1 (+)-CYANIDANOL-3 1 (+)-EPIPINORESINOL 1 (+)-EUDESMA-4(14),7(11)-DIENE-3-ONE 1 (+)-GALBACIN 2 (+)-GALLOCATECHIN 3 (+)-HERNANDEZINE 1 (+)-ISOCORYDINE 2 (+)-PSEUDOEPHEDRINE 1 (+)-SYRINGARESINOL 1 (+)-SYRINGARESINOL-DI-O-BETA-D-GLUCOSIDE 2 (+)-T-CADINOL 1 (+)-VESTITONE 1 (-)-16,17-DIHYDROXY-16BETA-KAURAN-19-OIC 1 (-)-3-HYDROXY-9-METHOXYPTEROCARPAN 1 (-)-ACANTHOCARPAN 1 (-)-ALPHA-BISABOLOL 2 (-)-ALPHA-HYDRASTINE 1 Chemical Activity Count (-)-APIOCARPIN 1 (-)-ARGEMONINE 1 (-)-BETONICINE 1 (-)-BISPARTHENOLIDINE 1 (-)-BORNYL-CAFFEATE 2 (-)-BORNYL-FERULATE 2 (-)-BORNYL-P-COUMARATE 2 (-)-CANESCACARPIN 1 (-)-CENTROLOBINE 1 (-)-CLANDESTACARPIN 1 (-)-CRISTACARPIN 1 (-)-DEMETHYLMEDICARPIN 1 (-)-DICENTRINE 1 (-)-DOLICHIN-A 1 (-)-DOLICHIN-B 1 (-)-EPIAFZELECHIN 2 (-)-EPICATECHIN 6 (-)-EPICATECHIN-3-O-GALLATE 2 (-)-EPICATECHIN-GALLATE 1 (-)-EPIGALLOCATECHIN 4 (-)-EPIGALLOCATECHIN-3-O-GALLATE 1 (-)-EPIGALLOCATECHIN-GALLATE 9 (-)-EUDESMIN 1 (-)-GLYCEOCARPIN 1 (-)-GLYCEOFURAN 1 (-)-GLYCEOLLIN-I 1 (-)-GLYCEOLLIN-II 1 2 Chemical Activity Count (-)-GLYCEOLLIN-III 1 (-)-GLYCEOLLIN-IV 1 (-)-GLYCINOL 1 (-)-HYDROXYJASMONIC-ACID 1 (-)-ISOSATIVAN 1 (-)-JASMONIC-ACID 1 (-)-KAUR-16-EN-19-OIC-ACID 1 (-)-MEDICARPIN 1 (-)-VESTITOL 1 (-)-VESTITONE 1 -

102 the Effects of Intravesical
102 Harper M, Brady C. M, Scaravilli F, Fowler C. J. Institute of Neurology, London THE EFFECTS OF INTRAVESICAL RESINIFERATOXIN ON SUBUROTHELIAL NERVE DENSITY IN PATIENTS WITH NEUROGENIC DETRUSOR OVERACTIVITY Aims of Study Somatic and autonomic neural pathways integrated in the CNS regulate urine storage and voiding. When spinal cord lesions disrupt these pathways, an aberrant segmental sacral reflex emerges, the afferent limb of which is comprised of small unmyelinated C-fibres that originate in the suburothelium. This pathological reflex is thought to be the underlying cause of “spinal” neurogenic detrusor overactivity (NDO). We are currently evaluating the role of resiniferatoxin (RTX) in the treatment of refractory “spinal” NDO. RTX is an ultra-potent capsaicin analogue purified from the dried latex of the succulent plant Euphorbia Resinifera. The vanillyl group of RTX facilitates the action of the vanilloid receptor (VR-1), a non-selective cation channel expressed on suburothelial C-fibres. Intravesical therapy with a single dose of RTX results in early acute excitatory effects (“sensitisation”) followed by inhibition of neuropeptide release and neuronal terminal field degeneration (“desensitisation”) (Avelino et al., 2001). In patients with refractory NDO, it has been shown that intravesical therapy with vanilloid compounds such as RTX or capsaicin significantly decreases lower urinary tract symptoms for a period of weeks or months, depending on factors such as dose and disease progression. It is thought that the eventual recurrence of symptoms is related to C-fibre regeneration. We have previously shown that the mean suburothelial nerve density decreases in patients who respond to capsaicin. Our experimental aim is to measure suburothelial nerve density in flexible cystoscopic biopsies in controls and patients with NDO before and after intravesical RTX or placebo. -

TRP CHANNELS AS THERAPEUTIC TARGETS TRP CHANNELS AS THERAPEUTIC TARGETS from Basic Science to Clinical Use
TRP CHANNELS AS THERAPEUTIC TARGETS TRP CHANNELS AS THERAPEUTIC TARGETS From Basic Science to Clinical Use Edited by ARPAD SZALLASI MD, PHD Department of Pathology, Monmouth Medical Center, Long Branch, NJ, USA AMSTERDAM • BOSTON • HEIDELBERG • LONDON NEW YORK • OXFORD • PARIS • SAN DIEGO SAN FRANCISCO • SINGAPORE • SYDNEY • TOKYO Academic Press is an imprint of Elsevier Academic Press is an imprint of Elsevier 125 London Wall, London, EC2Y 5AS, UK 525 B Street, Suite 1800, San Diego, CA 92101-4495, USA 225 Wyman Street, Waltham, MA 02451, USA The Boulevard, Langford Lane, Kidlington, Oxford OX5 1GB, UK First published 2015 Copyright © 2015 Elsevier Inc. All rights reserved. No part of this publication may be reproduced or transmitted in any form or by any means, electronic or mechanical, including photocopying, recording, or any information storage and retrieval system, without permission in writing from the publisher. Details on how to seek permission, further information about the Publisher’s permissions policies and our arrangement with organizations such as the Copyright Clearance Center and the Copyright Licensing Agency, can be found at our website: www.elsevier.com/permissions This book and the individual contributions contained in it are protected under copyright by the Publisher (other than as may be noted herein). Notices Knowledge and best practice in this field are constantly changing. As new research and experience broaden our understanding, changes in research methods, professional practices, or medical treatment may become necessary. Practitioners and researchers must always rely on their own experience and knowledge in evaluating and using any information, methods, compounds, or experiments described herein. -

TRPV1 and Endocannabinoids: Emerging Molecular Signals That Modulate Mammalian Vision
Cells 2014, 3, 914-938; doi:10.3390/cells3030914 OPEN ACCESS cells ISSN 2073-4409 www.mdpi.com/journal/cells Review TRPV1 and Endocannabinoids: Emerging Molecular Signals that Modulate Mammalian Vision 1,2,†, 1,2,† 1,† 1,2,3,4, Daniel A. Ryskamp *, Sarah Redmon , Andrew O. Jo and David Križaj * 1 Department of Ophthalmology & Visual Sciences, Moran Eye Institute, University of Utah School of Medicine, Salt Lake City, UT 84132, USA; E-Mails: [email protected] (S.R.); [email protected] (A.O.J.) 2 Interdepartmental Program in Neuroscience, University of Utah School of Medicine, Salt Lake City, UT 84132, USA 3 Department of Neurobiology & Anatomy, University of Utah School of Medicine, Salt Lake City, UT 84132, USA 4 Center for Translational Medicine, University of Utah School of Medicine, Salt Lake City, UT 84132, USA † Those authors contributed equally to this work. * Authors to whom correspondence should be addressed; E-Mails: [email protected] (D.A.R.); [email protected] (D.K.); Tel.: +1-801-213-2777 (D.K.); Fax: +1-801-587-8314 (D.K.). Received: 1 July 2014; in revised form: 27 August 2014 / Accepted: 5 September 2014 / Published: 12 September 2014 Abstract: Transient Receptor Potential Vanilloid 1 (TRPV1) subunits form a polymodal cation channel responsive to capsaicin, heat, acidity and endogenous metabolites of polyunsaturated fatty acids. While originally reported to serve as a pain and heat detector in the peripheral nervous system, TRPV1 has been implicated in the modulation of blood flow and osmoregulation but also neurotransmission, postsynaptic neuronal excitability and synaptic plasticity within the central nervous system. -

THE BIOLOGY, ECOLOGY and CONSERVATION of Euphorbia Clivicola in the LIMPOPO PROVINCE, SOUTH AFRICA
THE BIOLOGY, ECOLOGY AND CONSERVATION OF Euphorbia clivicola IN THE LIMPOPO PROVINCE, SOUTH AFRICA MASTER OF SCIENCE IN BOTANY S.I. CHUENE 2016 THE BIOLOGY, ECOLOGY AND CONSERVATION OF Euphorbia clivicola IN THE LIMPOPO PROVINCE, SOUTH AFRICA BY SELOBA IGNITIUS CHUENE A DISSERTATION SUBMITTED IN FULFILMENT FOR THE DEGREE OF MASTER OF SCIENCE IN BOTANY FACULTY OF SCIENCE AND AGRICULTURE, SCHOOL OF MOLECULAR AND LIFE SCIENCES, DEPARTMENT OF BIODIVERSITY AT THE UNIVERSITY OF LIMPOPO SUPERVISOR: PROF. M.J. POTGIETER CO-SUPERVISOR: MR. J.W. KRUGER (LEDET) 2016 LIMPOP OF O U TY NIVERSI Faculty of Science and Agriculture ABSTRACT The need to conduct a detailed biological and ecological study on Euphorbia clivicola was sparked by the drastic decline in the sizes of the Percy Fyfe Nature Reserve (Mokopane) and Radar Hill (Polokwane) populations, coupled with the discovery of two new populations; one in Dikgale and another in Makgeng village. The two newly (2012) discovered populations lacked scientific data necessary to develop an adaptive management plan. This study aimed to conduct a detailed biological and ecological assessment, in order to develop an informed management and monitoring plan for the four populations of E. clivicola. This study entailed a demographic investigation of all populations and an inter- population genetic diversity comparison so as to establish the relationship between all populations of E. clivicola. The abiotic and biotic interactions of E. clivicola were examined to determine the intrinsic and extrinsic factors causing the decline in the Percy Fyfe Nature Reserve and Radar Hill population sizes. Fire as one of the abiotic factors was observed to be beneficial to E. -

Osher Mini Medical School Feb 10 2016 Schumacher HAND OUT.Pptx
2/10/16! Back to the Future of Pain Medicine ! What is Pain?! ! Pain: “An unpleasant sensory and emotional Mark Schumacher Ph.D.,M.D.! experience associated with actual or potential Professor and Chief, Division of Pain Medicine! tissue damage, or described in terms of such Dept. of Anesthesia & Perioperative Care! damage (IASP).”! Medical Director, UCSF Pain Services! University of California, San Francisco! Plant derivatives imitate endogenous Have we made any progress in the analgesic systems pharmacologic treatment of pain?! ! Morphine! " 1805 - Primary therapy for severe pain is:! " Morphine! ! Endorphin! " 2016 - Primary therapy for severe pain is:! " Morphine! •" Sustained Release! Ideal analgesic ?! A revolution is underway in our understanding of the pain pathway! Acts selectively on the “pain-sensing” nerves! Does not depress CNS - respiration! .. and its origin began thousands of Use over time maintains analgesia! years ago with the physicians of ancient times! Easy to administer! Is not addictive! Affordable $$! ! 1! 2/10/16! # Anti-Atlas Mountains-Morocco! Capsaicin vs. Resiniferatoxin (RTX)! ! ! ! ! # Euphorbia Resinifera! Share a Common Modification ! Perception! Peppers Sail the Ocean Blue! Transmission! 2000 BC 63 BC 1428 1492 Euphobia Resinifera Transduction! Descartes 1644 ! Sherrington and the development ! of modern neurobiology - nociceptor! Primary Afferent Nociceptors! Sherrington (1906) ... Proposed: the experience of pain is based on nerves that responded to specific 2000 BC 63 BC 1428 1492 1906 noxious stimuli -

Baja California, Mexico, and a Vegetation Map of Colonet Mesa Alan B
Aliso: A Journal of Systematic and Evolutionary Botany Volume 29 | Issue 1 Article 4 2011 Plants of the Colonet Region, Baja California, Mexico, and a Vegetation Map of Colonet Mesa Alan B. Harper Terra Peninsular, Coronado, California Sula Vanderplank Rancho Santa Ana Botanic Garden, Claremont, California Mark Dodero Recon Environmental Inc., San Diego, California Sergio Mata Terra Peninsular, Coronado, California Jorge Ochoa Long Beach City College, Long Beach, California Follow this and additional works at: http://scholarship.claremont.edu/aliso Part of the Biodiversity Commons, Botany Commons, and the Ecology and Evolutionary Biology Commons Recommended Citation Harper, Alan B.; Vanderplank, Sula; Dodero, Mark; Mata, Sergio; and Ochoa, Jorge (2011) "Plants of the Colonet Region, Baja California, Mexico, and a Vegetation Map of Colonet Mesa," Aliso: A Journal of Systematic and Evolutionary Botany: Vol. 29: Iss. 1, Article 4. Available at: http://scholarship.claremont.edu/aliso/vol29/iss1/4 Aliso, 29(1), pp. 25–42 ’ 2011, Rancho Santa Ana Botanic Garden PLANTS OF THE COLONET REGION, BAJA CALIFORNIA, MEXICO, AND A VEGETATION MAPOF COLONET MESA ALAN B. HARPER,1 SULA VANDERPLANK,2 MARK DODERO,3 SERGIO MATA,1 AND JORGE OCHOA4 1Terra Peninsular, A.C., PMB 189003, Suite 88, Coronado, California 92178, USA ([email protected]); 2Rancho Santa Ana Botanic Garden, 1500 North College Avenue, Claremont, California 91711, USA; 3Recon Environmental Inc., 1927 Fifth Avenue, San Diego, California 92101, USA; 4Long Beach City College, 1305 East Pacific Coast Highway, Long Beach, California 90806, USA ABSTRACT The Colonet region is located at the southern end of the California Floristic Province, in an area known to have the highest plant diversity in Baja California. -

The Response of Cyanogenic and Acyanogenic Phenotypes of Trifolium Repens to Soil Moisture Supply W
THE RESPONSE OF CYANOGENIC AND ACYANOGENIC PHENOTYPES OF TRIFOLIUM REPENS TO SOIL MOISTURE SUPPLY W. FOULDS Science Department, Dudley CollegeofEducation and J. P. GRIME DepartmentofBotany, University of Sheffield Received14.vi.71 I. INTRODUCTION THE legumes Trjfolium repens L. and LotuscorniculatusL. are represented in many parts of the world by populations in which some or all of the plants are capable of releasing hydrocyanic acid under certain conditions. For both species, there is evidence (Barber, 1955; Jones, 1962) that cyanogenic individuals are less susceptible to grazing by small herbivores. Cyanogenesis is determined by two independent genes designated Ac and Li (Corkill, 1942; Atwood and Sullivan, 1943). The gene Ac is dominant and is responsible for the production of two cyanogenic glucosides, lotaus- tralin and linamarin, which occur in the proportion 4 : 1 (Melville and Doak, 1940). Modifying genes determine the quantity of glucoside produced (Corkill, 1940). The cyanogenic glucosides can be hydrolysed by the - glucosidase,linamarase (Coop, 1940) the production of which is governed by the dominant gene Li. Hydrolysis yields acetone (from linamarin), methyl-ethyl-ketone (from lotaustralin), glucose, hydrogen cyanide and water. Hughes (1968) maintains that two -glucosidases occur in Trjfolium repens, one, linamarase of high activity, the other of low activity. Corkill (1940) showed that individual plants may contain both enzyme and glucoside (AcLi), enzyme only (acLi), glucoside only (Acli) or neither (acli). Daday (1954a, b, 1958) measured the contribution of each of the four phenotypes to populations of Trfo1ium repens sampled on a world scale and found that high frequencies of both Ac and Li genes were associated with warm winter conditions. -

Connoisseurs' Cacti
ThCe actus Explorer The first free on-line Journal for Cactus and Succulent Enthusiasts 1 Siccobaccatus 2 Morangaya pensilis Number 17 3 Espostoa in Tenerife ISSN 2048-0482 4 Barranco Rambla de Ruiz December 2016 5 Juab and Utah County The Cactus Explorer ISSN 2048-0482 Number 17 December2016 IN THIS EDITION Regular Features Articles Introduction 3 A naturalised population of News and Events 4 Espostoa melanostele on Tenerife 21 In the Glasshouse 9 Travel with the cactus expert (16) 25 Journal Roundup 14 Where lizards dare: an excursion to Barranco On-line Journals 15 Rambla de Ruiz (Tenerife) 29 The Love of Books 18 Juab and Utah County, Utah, throughout the Society Pages 51 year 2015 36 Plants and Seeds for Sale 55 A Happy Medium? Morangaya pensilis . 41 Books for Sale 62 Cover Picture: Siccobaccatus dolichospermaticus See page 9 The No.1 source for on-line information about cacti and succulents is http://www.cactus-mall.com The best on-line library of succulent literature can be found at: https://www.cactuspro.com/biblio/en:accueil Invitation to Contributors Please consider the Cactus Explorer as the place to publish your articles. We welcome contributions for any of the regular features or a longer article with pictures on any aspect of cacti and succulents. The editorial team is happy to help you with preparing your work. Please send your submissions as plain text in a ‘Word’ document together with jpeg or tiff images with the maximum resolution available. A major advantage of this on-line format is the possibility of publishing contributions quickly and any issue is never full! We aim to publish your article quickly and the copy deadline is just a few days before the publication date.