Product List 2021 Reference Substances Natural Compounds
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
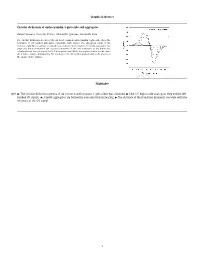
Graphical Abstract Circular Dichroism of Anthocyanidin 3-Glucoside Self
Graphical abstract Circular dichroism of anthocyanidin 3-glucoside self-aggregates Raquel Gavara, Vesselin Petrov, Alexandre Quintas, Fernando Pina * The circular dichroism spectra of the six most common anthocyanidin 3-glucoside show the formation of left handed aggregates compatible with dimers. The absorption bands of the monomer split by increasing concentration according to the formation of H and J aggregates. The angle and distance between the transition moments of the two monomers in the dimer was calculated from the splitting of the 0–0 absorption band. While the angle is similar for the series the distance changes dramatically. The intensity of the CD signal is proportional to the inverse of the square of the distance. Highlights Q10 " The circular dichroism spectra of six common anthocyanins 3-glucosides was obtained. " Like 3,5-diglucoside analogous they exhibit left- handed CD signals. " J and H aggregates are formed by concentration increasing. " The distance of the transition moments correlate with the intensity of the CD signal. 1 1 2 Circular dichroism of anthocyanidin 3-glucoside self-aggregates a a b a,⇑ 3 Q1 Raquel Gavara , Vesselin Petrov , Alexandre Quintas , Fernando Pina 4 a REQUIMTE, Departamento de Química, Faculdade de Ciências e Tecnologia, Universidade Nova de Lisboa, 2829516 Monte de Caparica, Portugal 5 b Instituto Superior de Ciencias da Saude Egas Moniz, Centro de Investigação Interdisciplinar Egas Moniz, P-2829511 Monte de Caparica, Caparica, Portugal 6 7 article info a b s t r a c t 1 9 10 Self-association constants for the flavylium cations of the six most common anthocyanidin 3-glucosides 20 11 were determined by circular dichroism (CD) and UV–Vis spectroscopy. -

Therapeutic Potential of Flavonoids in Inflammatory Bowel Disease: a Comprehensive Review
Submit a Manuscript: http://www.f6publishing.com World J Gastroenterol 2017 July 28; 23(28): 5097-5114 DOI: 10.3748/wjg.v23.i28.5097 ISSN 1007-9327 (print) ISSN 2219-2840 (online) REVIEW Therapeutic potential of flavonoids in inflammatory bowel disease: A comprehensive review Ali Salaritabar, Behrad Darvishi, Farzaneh Hadjiakhoondi, Azadeh Manayi, Antoni Sureda, Seyed Fazel Nabavi, Leo R Fitzpatrick, Seyed Mohammad Nabavi, Anupam Bishayee Ali Salaritabar, Behrad Darvishi, Department of Integrative work non-commercially, and license their derivative works on Oncology, Breast Cancer Research Center, Motamed Cancer different terms, provided the original work is properly cited and Institute, ACECR, Tehran 15179-64311, Iran the use is non-commercial. See: http://creativecommons.org/ licenses/by-nc/4.0/ Ali Salaritabar, Behrad Darvishi, Department of Recombinant Protein, Breast Cancer Research Center, Motamed Cancer Manuscript source: Invited manuscript Institute, ACECR, Tehran 15179-64311, Iran Correspondence to: Anupam Bishayee, PhD, Department Farzaneh Hadjiakhoondi, Azadeh Manayi, Medicinal Plants of Pharmaceutical Sciences, College of Pharmacy, Larkin Research Center, Faculty of Pharmacy, Tehran University of University, Miami, FL 33169, Medical Sciences, Tehran 14176-14411, Iran United States. [email protected] Telephone: +1-305-7607511 Antoni Sureda, Research Group on Community Nutrition and Oxidative Stress and CIBEROBN - Physiopathology of Obesity Received: February 16, 2017 and Nutrition, University of Balearic Islands, Palma -

(Roxb.) Craib and Kadsura Coccinea (Lem.) AC
foods Article Phenolic Profiles, Antioxidant, and Inhibitory Activities of Kadsura heteroclita (Roxb.) Craib and Kadsura coccinea (Lem.) A.C. Sm. Varittha Sritalahareuthai 1, Piya Temviriyanukul 1,2 , Nattira On-nom 1,2 , Somsri Charoenkiatkul 1 and Uthaiwan Suttisansanee 1,2,* 1 Institute of Nutrition, Mahidol University, Salaya, Phuttamonthon, Nakhon Pathom 73170, Thailand; [email protected] (V.S.); [email protected] (P.T.); [email protected] (N.O.-n.); [email protected] (S.C.) 2 Food and Nutrition Academic and Research Cluster, Institute of Nutrition, Mahidol University, Salaya, Phuttamonthon, Nakhon Pathom 73170, Thailand * Correspondence: [email protected]; Tel.: +66-(0)2800-2380 (ext. 422) Received: 4 August 2020; Accepted: 31 August 2020; Published: 2 September 2020 Abstract: Kadsura spp. in the Schisandraceae family are woody vine plants, which produce edible red fruits that are rich in nutrients and antioxidant activities. Despite their valuable food applications, Kadsura spp. are only able to grow naturally in the forest, and reproduction handled by botanists is still in progress with a very low growth rate. Subsequently, Kadsura spp. were listed as endangered species by the International Union for Conservation of Nature and Natural Resources (IUCN) in 2011. Two different Kadsura spp., including Kadsura coccinea (Lem.) A.C. Sm. and Kadsura heteroclita (Roxb.) Craib, are mostly found in northern Thailand. These rare, wild fruits are unrecognizable to outsiders, and there have only been limited investigations into its biological properties. This study, therefore, aimed to comparatively investigate the phenolic profiles, antioxidant activities, and inhibitory activities against the key enzymes involved in diabetes (α-glucosidase and α-amylase) and Alzheimer’s disease (acetylcholinesterase (AChE), butyrylcholinesterase (BChE), and beta-secretase 1 (BACE-1)) in different fruit parts (exocarp, mesocarp (edible part), seed, and core) of Kadsura coccinea (Lem.) A.C. -

Alteration of Anthocyanin Glycosylation in Cranberry Through Interspecific Hybridization
J. AMER. Soc. HORT. Sci. 130(5):711-715. 2005. Alteration of Anthocyanin Glycosylation in Cranberry Through Interspecific Hybridization Nicholi Vorsa Philip E. Marucci Center for Blueberry and Cranberry Research and Extension, Rutgers University, 125A Lake Oswego Road, Chatsworth, NJ 08019 James J. Polashock1 USDA—ARS Fruit Lab, 125A Lake Oswego Road, Chatsworth, NJ 08019 ADDITIONAL INDEX WORDS. Vaccinium macrocarpon, Vaccinium oxycoccus, antioxidant, bioavailability, flavonoid ABSTRACT. The flavonoids of american cranberry (Vaccinium macrocarpon Alt.) are documented to be beneficial for hu- man health. Among their benefits is a high antioxidant potential, with anthocyanin glycosides being the main contribu- tors. Flavonoid glucose conjugates are reported to be more bioavailable than those with other sugar conjugates. The anthocyanin glycosides of V. macrocarpon fruit are mainly galactosides and arabinosides of the aglycones, cyanidin and peonidin, with less than 8% glucosides. In contrast, the fruit anthocyanins of another cranberry species, V. oxycoccus L. were found to be largely glucosides of cyanidin and peonidin. Interspecific hybrids between these two species were intermediate to the parental species in the proportion of fruit anthocyanin glucosides. About half the progeny (1:1 segregation) in a backcross population (to V. macrocarpon) maintained the relatively high anthocyanin glucoside ratio. In this study, we demonstrate the genetic manipulation of anthocyanin glycosylation in cranberry using interspecific hybridization, resulting in dramatically increased glucose-conjugated anthocyanins. Flavonoids are considered to be secondary metabolites, which The cultivated american cranberry (V. macrocarpon) is recog- have been associated with roles in ultraviolet protection, plant nized for its brilliant red fruit due to an abundance of anthocyanins sexual reproduction, pollinator attraction, symbiotic plant—microbe in the fruit epidermal tissues. -

Graphical Abstract CG 18-1-MS
Send Orders for Reprints to [email protected] 3 Current Genomics, 2017, 18, 3-26 REVIEW ARTICLE ISSN: 1389-2029 eISSN: 1875-5488 Impact Factor: 2.43 Established Human Cell Lines as Models to Study Anti-leukemic Effects of Flavonoids BENTHAM SCIENCE Katrin Sak* and Hele Everaus Department of Hematology and Oncology, University of Tartu, Tartu, Estonia Abstract: Despite the extensive work on pathological mechanisms and some recent advances in the treatment of different hematological malignancies, leukemia continues to present a significant challenge being frequently considered as incurable disease. Therefore, the development of novel therapeutic agents with high efficacy and low toxicity is urgently needed to improve the overall survival rate of pa- A R T I C L E H I S T O R Y tients. In this comprehensive review article, the current knowledge about the anticancer activities of Received: May 11, 2015 flavonoids as plant secondary polyphenolic metabolites in the most commonly used human established Revised: November 20, 2015 Accepted: November 27, 2015 leukemia cell lines (HL-60, NB4, KG1a, U937, THP-1, K562, Jurkat, CCRF- CEM, MOLT-3, and MOLT-4) is compiled, revealing clear anti-proliferative, pro-apoptotic, cell cycle arresting, and differ- DOI: 10.2174/138920291766616080316 entiation inducing effects for certain compounds. Considering the low toxicity of these substances in 5447 normal blood cells, the presented data show a great potential of flavonoids to be developed into novel anti-leukemia agents applicable also in the malignant cells resistant to the current conventional che- motherapeutic drugs. Keywords: Antiproliferation, Apoptosis, Cell cycle arrest, Cytotoxicity, Differentiation, Flavonoids, Leukemia, Human cell lines. -

Comparative Studies of Corrosion Inhibitive Properties of Benzofuran
ISSN (Online) 2456-1290 International Journal of Engineering Research in Mechanical and Civil Engineering (IJERMCE) Vol 2, Issue 11,November 2017 Comparative studies of Corrosion Inhibitive Properties of Benzofuran-2-carboxylic acid & Amla Leaves Extract On Mild Steel in Acid Media [1] Abhishek Kumar, [2] Ankit Aggarwal, [3] Ashutosh Krishna Piyush, [4] Abhishek Kumar, [5] Aatiq ShafiqDar, [6] Angel Roy, [7] Dr Hari Krishna S [1][2][3][6] 1st Year Department Of Computer Science & Engineering, Sri SaiRam College Of Engineering, Bangalore, [4] 1st Year Department of Electrical & Electronics, Sri SaiRam College Of Engineering, Bangalore [5] 1st Year Department Of Mechanical Engineering, Sri SaiRam College Of Engineering, Bangalore [7] Assistant Professor, Department Of Chemistry, Sri SaiRam College Of Engineering, Bangalore Abstract:- The effects of Benzofuran-2-carboxylic acid (BF) & Amla (Emblica officinal is) leaves aqueous extract as a corrosion inhibitor, behaviour of mild steel has been investigated in hydrochloric acid solutions containing Experiments were performed by weight loss method for different time intervals and at room temperature. The inhibition efficiency of Benzofuran-2-carboxylic acid was found to increase with inhibitor concentration and also in the presence of sodium bromide and sodium iodide. Inhibition efficiency was found to increase with increasing concentration of inhibitor (0.2 g /l to 10 g/l) for 6 hours at room temperature. The maximum inhibition efficiency of Emblica officinal is leaving 87 % in 1 N Hydrochloric acid. From the comparative studies, it was investigated that the corrosion inhibition efficiency of Emblica officinal is leaving as aqueous extract is approximately equal to that of Benzofuran-2-carboxylic acid in hydrochloric acid. -

Cytotoxic Activities of Flavonoids from Centaurea Scoparia
Hindawi Publishing Corporation e Scientific World Journal Volume 2014, Article ID 274207, 7 pages http://dx.doi.org/10.1155/2014/274207 Research Article Cytotoxic Activities of Flavonoids from Centaurea scoparia Sayed A. Ahmed and Emadeldin M. Kamel Chemistry Department, Faculty of Science, Beni Suef University, Salah Salem Street, P.O. Box 62514, Beni Suef 62514, Egypt Correspondence should be addressed to Sayed A. Ahmed; [email protected] Received 17 January 2014; Accepted 22 May 2014; Published 11 June 2014 Academic Editor: Diego Savoia Copyright © 2014 S. A. Ahmed and E. M. Kamel. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Phytochemical studies on the ethanolic extract of the aerial parts of Centaurea scoparia ledtotheisolationof two new flavonoids, 3 ,4 -dihydroxy-(3 ,4 -dihydro-3 -hydroxy-4 -acetoxy)-2 ,2 -dimethylpyrano-(5 ,6 :7,8)-flavone-3-O-- D-glucopyranoside (1)and3,3,4 -trihydroxy-(3 ,4 -dihydro-3 ,4 -dihydroxy)-2 ,2 -dimethylpyrano-(5 ,6 :7,8)-flavone (2), along with eight known flavonoids isolated for the first time from this plant, cynaroside (3), Apigetrin (4), centaureidin (5), oroxylin A(6), 5,7-dihydroxy-3 ,4 ,5 -trimethoxyflavone (7), atalantoflavone (8), 5-hydroxy-3 ,4 ,8-trimethoxy-2 ,2 -dimethylpyrano (5 ,6 :6,7)-flavone (9), and 3 ,4 ,5,8-tetramethoxy-2 ,2 -dimethylpyrano (5 ,6 :6,7)-flavone (10). The structures of the isolated compounds were elucidated by means of spectroscopic tools including 1D and 2D NMR, UV,IR, and mass spectroscopy. -

Current Perspectives on Medicinal and Aromatic Plants
Review Curr. Pers. MAPs, (2021); 4(1): 66-86 Current Perspectives on Medicinal and Aromatic Plants An International Journal ISSN: 2619-9645 | e-ISSN: 2667-5722 Prevention of Viral Effect and Enhancement of Immune System with the help of Herbal Plants and Himalayan Crude Drugs in SAR-COV-2 Patient: A Review Rishiram BARAL1,2 1Department of Pharmaceutical Sciences, School of Health and Allied Sciences, Faculty of Health Science, Pokhara University, Pokhara, Nepal 2Research Institute of Pharmaceutical Sciences, Department of Pharmacy, Kyungpook National University, Daegu, South Korea *Corresponding author: [email protected] Received : 07/06/2021 https://doi.org/ 10.38093/cupmap.948975 Accepted : 30/06/2021 Abstract After December 2019, Severe Acute Respiratory Syndrome (SAR-COV-2) become a life-threatening issue to the entire human society when it started to spread exponentially all over the world from Wuhan city of China. The virus directly hits the upper and lower respiratory tract of human airways and causes severe damage to the human lung, leading to multiorgan failure, hypoxemia, and dyspnea. Similarly, studies revealed that SAR-COV-2 severely hit the younger and aged population in which the immune system is seriously compromised. The cell of the immune system such as T-cells, B-cells, NK cells, etc. helps to fight against such viral antigen and resist critical viral damage. Therefore, enhancement of the immune system could also be an effective approach to prevent viral infection and even aid in the reduction of the death count. Nowadays, dietary, and herbal remedies are being integrated into the mainstream of the healthcare systems because of their multi-ingredient character, and some of them are known to render efficacy comparable to that of synthetic drug substances. -

In Vitro Bioaccessibility, Human Gut Microbiota Metabolites and Hepatoprotective Potential of Chebulic Ellagitannins: a Case of Padma Hepatenr Formulation
Article In Vitro Bioaccessibility, Human Gut Microbiota Metabolites and Hepatoprotective Potential of Chebulic Ellagitannins: A Case of Padma Hepatenr Formulation Daniil N. Olennikov 1,*, Nina I. Kashchenko 1,: and Nadezhda K. Chirikova 2,: Received: 28 August 2015 ; Accepted: 30 September 2015 ; Published: 13 October 2015 1 Laboratory of Medical and Biological Research, Institute of General and Experimental Biology, Siberian Division, Russian Academy of Science, Sakh’yanovoy Street 6, Ulan-Ude 670-047, Russia; [email protected] 2 Department of Biochemistry and Biotechnology, North-Eastern Federal University, 58 Belinsky Street, Yakutsk 677-027, Russian; [email protected] * Correspondence: [email protected]; Tel.: +7-9021-600-627; Fax: +7-3012-434-243 : These authors contributed equally to this work. Abstract: Chebulic ellagitannins (ChET) are plant-derived polyphenols containing chebulic acid subunits, possessing a wide spectrum of biological activities that might contribute to health benefits in humans. The herbal formulation Padma Hepaten containing ChETs as the main phenolics, is used as a hepatoprotective remedy. In the present study, an in vitro dynamic model simulating gastrointestinal digestion, including dialysability, was applied to estimate the bioaccessibility of the main phenolics of Padma Hepaten. Results indicated that phenolic release was mainly achieved during the gastric phase (recovery 59.38%–97.04%), with a slight further release during intestinal digestion. Dialysis experiments showed that dialysable phenolics were 64.11% and 22.93%–26.05% of their native concentrations, respectively, for gallic acid/simple gallate esters and ellagitanins/ellagic acid, in contrast to 20.67% and 28.37%–55.35% for the same groups in the non-dialyzed part of the intestinal media. -

(Lapageria Rosea) C
J. Chil. Chem. Soc., 54, Nº 2 (2009) ANTHOCYANINS THAT CONFER CHARACTERISTIC COLOR TO RED COPIHUE FLOWERS (LAPAGERIA ROSEA) C. VERGARA1, D. VON BAER1*, I. HERMOSÍN2, A. RUIZ1, M.A. HITSCHFELD1, N. CASTILLO2 AND C. MARDONES1. 1 Universidad de Concepción, Departamento de Análisis Instrumental, Facultad de Farmacia, Casilla 160 – C, Concepción, Chile. 2 Universidad de Castilla-La Mancha, Escuela Universitaria de Ingeniería Técnica Agrícola, Ronda de Calatrava 7, 13071 Ciudad Real, España. (Received: January 7, 2009 - Accepted: February 25, 2009) ABSTRACT The Copihue (Lapageria rosea), also known as the Chilean bellflower, is the national flower of Chile and is the only species in the genus Lapageria. The copihue’s tepals are commonly red, with white or pink being less common. The red color of the copihue has been glorified in legends, poems and popular songs. The present work studies the pigments that confer red copihues their characteristic color. The principal types of cyanidin present in red copihue’s tepals are cyanidin-3-O-rhamnosylglucoside, followed by cyanidin-3-O-glucoside, and while only the latter is detected in pink tepals and neither one are detected in white flowers. Based on the obtained results by HPLC-ESI-MSn and HPLC-DAD, it is concluded that rhamnosyl- and glucosyl-derivatives of cyanidin, which present respectively an absorption maximum at 518 and 516 nm, confer the characteristic red color to red copihues. Furthermore, glycosilated cyanidin derivatives, pigments derived from other anthocyanidins, were not detected in red copihue flowers even when they are present in other red flowering plants. Keywords: flower, copihue, Lapageria rosea, anthocyanins, cyanidin-3-O-rutinoside, cyanidin-3-O-glucoside. -

Ellagitannin–Lipid Interaction by HR-MAS NMR Spectroscopy
molecules Article Ellagitannin–Lipid Interaction by HR-MAS NMR Spectroscopy Valtteri Virtanen * , Susanna Räikkönen, Elina Puljula and Maarit Karonen Natural Chemistry Research Group, Department of Chemistry, University of Turku, FI-20014 Turku, Finland; [email protected] (S.R.); [email protected] (E.P.); maarit.karonen@utu.fi (M.K.) * Correspondence: vtjvir@utu.fi; Tel.: +358-29-450-3205 Abstract: Ellagitannins have antimicrobial activity, which might be related to their interactions with membrane lipids. We studied the interactions of 12 different ellagitannins and pentagalloylglucose with a lipid extract of Escherichia coli by high-resolution magic angle spinning NMR spectroscopy. The nuclear Overhauser effect was utilized to measure the cross relaxation rates between ellagitannin and lipid protons. The shifting of lipid signals in 1H NMR spectra of ellagitannin–lipid mixture due to ring current effect was also observed. The ellagitannins that showed interaction with lipids had clear structural similarities. All ellagitannins that had interactions with lipids had glucopy- ranose cores. In addition to the central polyol, the most important structural feature affecting the interaction seemed to be the structural flexibility of the ellagitannin. Even dimeric and trimeric ellagitannins could penetrate to the lipid bilayers if their structures were flexible with free galloyl and hexahydroxydiphenoyl groups. Keywords: E. coli; HR-MAS-NMR; interaction; lipid membrane; tannins; UPLC-DAD-MS Citation: Virtanen, V.; Räikkönen, S.; Puljula, E.; Karonen, M. 1. Introduction Ellagitannin–Lipid Interaction by HR-MAS NMR Spectroscopy. Tannins are a group of specialized plant metabolites, which, when included in the di- Molecules 2021, 26, 373. etary feed of ruminants, have been shown to induce many beneficial effects such as increas- https://doi.org/10.3390/ ing their effective amino acid absorption, lowering their methane production, and acting as molecules26020373 anthelmintics [1–6]. -

Effects of Anthocyanins on the Ahr–CYP1A1 Signaling Pathway in Human
Toxicology Letters 221 (2013) 1–8 Contents lists available at SciVerse ScienceDirect Toxicology Letters jou rnal homepage: www.elsevier.com/locate/toxlet Effects of anthocyanins on the AhR–CYP1A1 signaling pathway in human hepatocytes and human cancer cell lines a b c d Alzbeta Kamenickova , Eva Anzenbacherova , Petr Pavek , Anatoly A. Soshilov , d e e a,∗ Michael S. Denison , Michaela Zapletalova , Pavel Anzenbacher , Zdenek Dvorak a Department of Cell Biology and Genetics, Faculty of Science, Palacky University, Slechtitelu 11, 783 71 Olomouc, Czech Republic b Institute of Medical Chemistry and Biochemistry, Faculty of Medicine and Dentistry, Palacky University, Hnevotinska 3, 775 15 Olomouc, Czech Republic c Department of Pharmacology and Toxicology, Charles University in Prague, Faculty of Pharmacy in Hradec Kralove, Heyrovskeho 1203, Hradec Kralove 50005, Czech Republic d Department of Environmental Toxicology, University of California, Meyer Hall, One Shields Avenue, Davis, CA 95616-8588, USA e Institute of Pharmacology, Faculty of Medicine and Dentistry, Palacky University, Hnevotinska 3, 775 15 Olomouc, Czech Republic h i g h l i g h t s • Food constituents may interact with drug metabolizing pathways. • AhR–CYP1A1 pathway is involved in drug metabolism and carcinogenesis. • We examined effects of 21 anthocyanins on AhR–CYP1A1 signaling. • Human hepatocytes and cell lines HepG2 and LS174T were used as the models. • Tested anthocyanins possess very low potential for food–drug interactions. a r t i c l e i n f o a b s t r a c t