Signature Redacted for Privacy
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Exobasidium Darwinii, a New Hawaiian Species Infecting Endemic Vaccinium Reticulatum in Haleakala National Park
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Springer - Publisher Connector Mycol Progress (2012) 11:361–371 DOI 10.1007/s11557-011-0751-4 ORIGINAL ARTICLE Exobasidium darwinii, a new Hawaiian species infecting endemic Vaccinium reticulatum in Haleakala National Park Marcin Piątek & Matthias Lutz & Patti Welton Received: 4 November 2010 /Revised: 26 February 2011 /Accepted: 2 March 2011 /Published online: 8 April 2011 # The Author(s) 2011. This article is published with open access at Springerlink.com Abstract Hawaii is one of the most isolated archipelagos Exobasidium darwinii is proposed for this novel taxon. This in the world, situated about 4,000 km from the nearest species is characterized among others by the production of continent, and never connected with continental land peculiar witches’ brooms with bright red leaves on the masses. Two Hawaiian endemic blueberries, Vaccinium infected branches of Vaccinium reticulatum. Relevant char- calycinum and V. reticulatum, are infected by Exobasidium acters of Exobasidium darwinii are described and illustrated, species previously recognized as Exobasidium vaccinii. additionally phylogenetic relationships of the new species are However, because of the high host-specificity of Exobasidium, discussed. it seems unlikely that the species infecting Vaccinium calycinum and V. reticulatum belongs to Exobasidium Keywords Exobasidiomycetes . ITS . LSU . vaccinii, which in the current circumscription is restricted to Molecular phylogeny. Ustilaginomycotina -
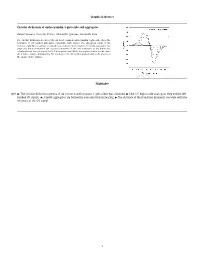
Graphical Abstract Circular Dichroism of Anthocyanidin 3-Glucoside Self
Graphical abstract Circular dichroism of anthocyanidin 3-glucoside self-aggregates Raquel Gavara, Vesselin Petrov, Alexandre Quintas, Fernando Pina * The circular dichroism spectra of the six most common anthocyanidin 3-glucoside show the formation of left handed aggregates compatible with dimers. The absorption bands of the monomer split by increasing concentration according to the formation of H and J aggregates. The angle and distance between the transition moments of the two monomers in the dimer was calculated from the splitting of the 0–0 absorption band. While the angle is similar for the series the distance changes dramatically. The intensity of the CD signal is proportional to the inverse of the square of the distance. Highlights Q10 " The circular dichroism spectra of six common anthocyanins 3-glucosides was obtained. " Like 3,5-diglucoside analogous they exhibit left- handed CD signals. " J and H aggregates are formed by concentration increasing. " The distance of the transition moments correlate with the intensity of the CD signal. 1 1 2 Circular dichroism of anthocyanidin 3-glucoside self-aggregates a a b a,⇑ 3 Q1 Raquel Gavara , Vesselin Petrov , Alexandre Quintas , Fernando Pina 4 a REQUIMTE, Departamento de Química, Faculdade de Ciências e Tecnologia, Universidade Nova de Lisboa, 2829516 Monte de Caparica, Portugal 5 b Instituto Superior de Ciencias da Saude Egas Moniz, Centro de Investigação Interdisciplinar Egas Moniz, P-2829511 Monte de Caparica, Caparica, Portugal 6 7 article info a b s t r a c t 1 9 10 Self-association constants for the flavylium cations of the six most common anthocyanidin 3-glucosides 20 11 were determined by circular dichroism (CD) and UV–Vis spectroscopy. -

In Vitro Fermentation Characteristics and Methane Mitigation Responded to Flavonoid Extract Levels from Alternanthera Sissoo and Dietary Ratios
fermentation Article In Vitro Fermentation Characteristics and Methane Mitigation Responded to Flavonoid Extract Levels from Alternanthera sissoo and Dietary Ratios Sukruthai Sommai 1, Anusorn Cherdthong 1,* , Chanon Suntara 1, Sarong So 1, Metha Wanapat 1 and Sineenart Polyorach 2 1 Tropical Feed Resources Research and Development Center (TROFREC), Department of Animal Science, Faculty of Agriculture, Khon Kaen University, Khon Kaen 40002, Thailand; [email protected] (S.S.); [email protected] (C.S.); [email protected] (S.S.); [email protected] (M.W.) 2 Department of Animal Production Technology and Fisheries, Faculty of Agricultural Technology, King Mongkut’s Institute of Technology, Bangkok 10520, Thailand; [email protected] * Correspondence: [email protected]; Tel.: +66-43-202-362; Fax: +66-43-202-362 Abstract: Two experiments were conducted under this study: Experiment 1 was to study production yield, chemical composition, and in vitro degradability of Brazilian spinach (Alternanthera sissoo; BS) leaf and leaf + leaf-stalk at various maturity ages of 15, 30, 45, and 60 days after plantation and regrowth and Experiment 2 was to evaluate the effect of flavonoid extract from BS leaf and leaf + leaf-stalk and dietary ratios on ruminal gas production, fermentation characteristics, and in vitro degradability. Experiment 1 showed that maturity ages after planting and regrowth increased, Citation: Sommai, S.; the yield significantly increased. Increasing maturity ages significantly (p < 0.05) increased neutral Cherdthong, A.; Suntara, C.; So, S.; detergent fiber and acid detergent fiber content and decreased crude protein content, total flavonoid Wanapat, M.; Polyorach, S. In Vitro (TF) content, and degradability for both leaf and leaf + leaf-stalk. -

(Vinca Rosea and Vinca Difformis) from Libya
Research Article Open Acc J of Toxicol Volume 4 Issue 1 - April 2019 Copyright © All rights are reserved by Salem Mohamed Edrah DOI : 10.19080/OAJT.2019.04.555626 Phytochemical Study and In Vitro Antibacterial Activity of Two Traditional Medicinal Plants (Vinca Rosea and Vinca Difformis) from Libya Salem Mohamed Edrah1*, Fatimh Mustafa Meelad1 and Fouzy Alafid2 1Department of Chemistry, Al-Khums El-Mergeb University, Libya 2University of Pardubice, Czech Republic Submission: March 04, 2019; Published: April 02, 2019 *Corresponding author: Salem Mohamed Edrah, Department of Chemistry, Faculty of Sciences Al-Khums El-Mergeb University, Al-Khums, Libya Abstract Vinca rosea and Vinca difformis gardens. Aqueous and Ethanol extracts leaf of both species were preliminary phytochemically screened and tested against some pathogenic bacteria. Qualitatively analysis revealed are magnificentTannin, Saponin, plants Flavonoids growing in and the Terpenoids Libyan woodlands gave positive and are results utilized and beautifully phlobatanins as an and embellishing Steroids gave in negative results. Quantitative analysis revealed that the percentage yield of bioactive constituents is present more in Vinca rosea than in Vinca difformis. In addition, the crude extracts of Vinca rosea and Vinca difformis were tested (using the Disc Diffusion Method) for their antimicrobial bacteria include Staphylococcus aureus and Streptococcus spp., and two strains of gram-negative bacteria including Escherichia coli and Klebsiella pneumoniaactivity against the bacterial pathogens. The influences of aqueous and ethanol extracts on some pathogenic: two strains of gram-positive antibiotics by measuring the diameter of the inhibition zones, After incubation, the zone of inhibition was measured in mm, a good inhibition of more than ,6 and mm the were sensitivity observed of indicating the microorganisms the effective to antibacterial the extracts activityof plant’s of thespecies bioactive were compoundscompared with in both each of other the plants and with leaves designated extracts. -

Tannins, Saponins and Calcium Oxalate Crystals from Nigerian Species of Boerhavia L
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Elsevier - Publisher Connector South African Journal of Botany 2002, 68: 386–388 Copyright © NISC Pty Ltd Printed in South Africa — All rights reserved SOUTH AFRICAN JOURNAL OF BOTANY ISSN 0254–6299 Short Communication Tannins, saponins and calcium oxalate crystals from Nigerian species of Boerhavia L. (Nyctaginaceae) HO Edeoga* and CI Ikem Department of Biological Sciences, Federal University of Agriculture, Umudike PMB 7267, Umuahia — Abia State, Nigeria * Corresponding author, e-mail: [email protected] Received 24 July 2001, accepted in revised form 19 October 2001 Three species of Boerhavia from southern Nigeria have tals were present around the regions of the cortex of the been screened phytochemically. The presence of tan- stem but were localised almost in every tissue of the nins and saponins are being reported for the first time root. Tannins and saponins were isolated from the tis- in B. coccinea and B. erecta whereas the report of these sues (leaves, stems and roots) of these plants hence the substances in B. diffusa corroborates previous reports. significance of these findings remains an area of further The nature of calcium oxalate crystals in the roots and investigations as far as the chemical constituents of stems of B. diffusa and B. erecta could be utilised for these plants are concerned. the characterisation of these plants. The oxalate crys- Boerhavia L. is a herbaceous dicotyledonous plant distrib- ferent families (Bohm and Koupai–Abyazani 1993, Touyama uted in the tropics and subtropics of the world. The West et al. -

Forest Pathology in Hawaii*
343 FOREST PATHOLOGY IN HAWAII* DONALD E. GARDNERt United States Geological Survey, Biological Resources Division, Pacific Island Ecological Research Center, Department of Botany, University of Hawaii at Manoa 3190 Maile Way, Honolulu, Hawaii 96822, USA (Revision received for publication 3 February 2004) ABSTRACT Native Hawaiian forests are characterised by a high degree of endemism, including pathogens as well as their hosts. With the exceptions of koa (Acacia koa Gray), possibly maile (Alyxia olivifonnis Gaud.), and, in the past, sandalwood (Santalum spp.), forest species are of little commercial value. On the other hand, these forests are immensely important from a cultural, ecological, and evolutionary standpoint. Forest disease research was lacking during the mid-twentieth century, but increased markedly with the recognition of ohia (Metrosideros polymorpha Gaud.) decline in the 1970s. Because many pathogens are themselves endemic, or are assumed to be, having evolved with their hosts, research emphasis in natural areas is on understanding host-parasite interactions and evolutionary influences, rather than disease control. Aside from management of native forests, attempts at establishing a commercial forest industry have included importation of several species of pine, Araucana, and Eucalyptus as timber crops, and of numerous ornamentals. Diseases of these species have been introduced with their hosts. The attacking of native species by introduced pathogens is problematic — for example, Armillaria mellea (Vahl ex Fr.) Quel, on koa and mamane (Sophora chrysophylla (Salisb.) Seem.). Much work remains to be done in both native and commercial aspects of Hawaiian forest pathology. Keywords: endemic species; indigenous species; introduced species; island ecology; ohia decline. INTRODUCTION AND HISTORY Forest pathology in Hawaii, as elsewhere, originated with casual observations of obvious disease and decline phenomena by amateur observers and general foresters. -

Guidance Document Pohakuloa Training Area Plant Guide
GUIDANCE DOCUMENT Recovery of Native Plant Communities and Ecological Processes Following Removal of Non-native, Invasive Ungulates from Pacific Island Forests Pohakuloa Training Area Plant Guide SERDP Project RC-2433 JULY 2018 Creighton Litton Rebecca Cole University of Hawaii at Manoa Distribution Statement A Page Intentionally Left Blank This report was prepared under contract to the Department of Defense Strategic Environmental Research and Development Program (SERDP). The publication of this report does not indicate endorsement by the Department of Defense, nor should the contents be construed as reflecting the official policy or position of the Department of Defense. Reference herein to any specific commercial product, process, or service by trade name, trademark, manufacturer, or otherwise, does not necessarily constitute or imply its endorsement, recommendation, or favoring by the Department of Defense. Page Intentionally Left Blank 47 Page Intentionally Left Blank 1. Ferns & Fern Allies Order: Polypodiales Family: Aspleniaceae (Spleenworts) Asplenium peruvianum var. insulare – fragile fern (Endangered) Delicate ENDEMIC plants usually growing in cracks or caves; largest pinnae usually <6mm long, tips blunt, uniform in shape, shallowly lobed, 2-5 lobes on acroscopic side. Fewer than 5 sori per pinna. Fronds with distal stipes, proximal rachises ocassionally proliferous . d b a Asplenium trichomanes subsp. densum – ‘oāli’i; maidenhair spleenwort Plants small, commonly growing in full sunlight. Rhizomes short, erect, retaining many dark brown, shiny old stipe bases.. Stipes wiry, dark brown – black, up to 10cm, shiny, glabrous, adaxial surface flat, with 2 greenish ridges on either side. Pinnae 15-45 pairs, almost sessile, alternate, ovate to round, basal pinnae smaller and more widely spaced. -

Compounds of Vaccinium Membranaceum and Vaccinium Ovatum Native to the Pacific Northwest of North America
J. Agric. Food Chem. 2004, 52, 7039−7044 7039 Comparison of Anthocyanin Pigment and Other Phenolic Compounds of Vaccinium membranaceum and Vaccinium ovatum Native to the Pacific Northwest of North America JUNGMIN LEE,† CHAD E. FINN,§ AND RONALD E. WROLSTAD*,† Department of Food Science, Oregon State University, Corvallis, Oregon 97331, and Northwest Center for Small Fruit Research, U.S. Department of Agriculture-Agricultural Research Service, HCRL, 3420 NW Orchard Avenue, Corvallis, Oregon 97330 Two huckleberry species, Vaccinium membranaceum and Vaccinium ovatum, native to Pacific Northwestern North America, were evaluated for their total, and individual, anthocyanin and polyphenolic compositions. Vaccinium ovatum had greater total anthocyanin (ACY), total phenolics (TP), oxygen radical absorbing capacity (ORAC), and ferric reducing antioxidant potential (FRAP) than did V. membranaceum. The pH and °Brix were also higher in V. ovatum. Berry extracts from each species were separated into three different fractionssanthocyanin, polyphenolic, and sugar/ acidsby solid-phase extraction. The anthocyanin fractions of each species had the highest amount of ACY, TP, and antioxidant activity. Each species contained 15 anthocyanins (galactoside, glucoside, and arabinoside of delphinidin, cyanidin, petunidin, peonidin, and malvidin) but in different proportions. Their anthocyanin profiles were similar by high-performance liquid chromatography with photodiode array detection (LC-DAD) and high-performance liquid chromatography with photodiode array and mass spectrometry detections (LC-DAD-MS). Each species had a different polyphenolic profile. The polyphenolics of both species were mainly composed of cinnamic acid derivatives and flavonol glycosides. The major polyphenolic compound in V. membranaceum was neochlorogenic acid, and in V. ovatum, chlorogenic acid. KEYWORDS: Vaccinium; huckleberry; anthocyanins; phenolics; antioxidant activity INTRODUCTION to Vaccinium consanguineum Klotsch, native to Central America, and Vaccinium floribundum Kunth, native to Andean S. -

Susceptibility of Some Native Plant Species from Hawaii to Phytophthora Ramorum 1
Proceedings of the Sudden Oak Death Third Science Symposium Susceptibility of Some Native Plant Species 1 From Hawaii to Phytophthora ramorum Paul Reeser,2 Everett Hansen,2 Wendy Sutton,2 Jennifer Davidson,3 and Jennifer Parke2 Abstract The remaining native flora of Hawaii are under continuing pressure from habitat loss and exotic, invasive organisms, including animals, plants, and pathogens. In order to assess the risk to P. ramorum, we inoculated seedlings of Metrosideros polymorpha (ohia), Vaccinium calycinum (ohelo), Acacia koa (koa), and Leptecophylla tameiameiae (pukiawe) with the pathogen. Two isolates were used, one from an Oregon horticultural nursery, and one from tanoak in the Oregon infested forest area. A zoospore suspension was sprayed on the intact plants. Plants were inspected periodically and symptomatic plants or plant parts were harvested and photographed. For isolation of P. ramorum symptomatic tissue was surface disinfested and plated in semi-selective agar. There was some symptom development in some inoculated plants for all the species tested. However, infection was confirmed by re-isolation of P. ramorum only in ohelo, pukiawe, and koa. Detached leaf inoculation also demonstrated susceptibility of Vaccinium reticulatum, another species of ohelo. Key words: Sudden oak death, Metrosideros polymorpha (ohia), Vaccinium calycinum (ohelo), Vaccinium reticulatum, Acacia koa (koa), and Leptecophylla tameiameiae (pukiawe). Materials and Methods Metrosideros polymorpha (ohia), Vaccinium calycinum (ohelo), Acacia koa (koa), and Leptecophylla tameiameiae (pukiawe) were inoculated with zoospore suspensions of Phytophthora. ramorum forest isolate 2027.1 (1.6 x 105 per ml) and nursery isolate 03-74-1 (9.5 x 104 per ml). Inoculum was applied to plants with an airbrush sprayer. -

Effects of Anthocyanins on the Ahr–CYP1A1 Signaling Pathway in Human
Toxicology Letters 221 (2013) 1–8 Contents lists available at SciVerse ScienceDirect Toxicology Letters jou rnal homepage: www.elsevier.com/locate/toxlet Effects of anthocyanins on the AhR–CYP1A1 signaling pathway in human hepatocytes and human cancer cell lines a b c d Alzbeta Kamenickova , Eva Anzenbacherova , Petr Pavek , Anatoly A. Soshilov , d e e a,∗ Michael S. Denison , Michaela Zapletalova , Pavel Anzenbacher , Zdenek Dvorak a Department of Cell Biology and Genetics, Faculty of Science, Palacky University, Slechtitelu 11, 783 71 Olomouc, Czech Republic b Institute of Medical Chemistry and Biochemistry, Faculty of Medicine and Dentistry, Palacky University, Hnevotinska 3, 775 15 Olomouc, Czech Republic c Department of Pharmacology and Toxicology, Charles University in Prague, Faculty of Pharmacy in Hradec Kralove, Heyrovskeho 1203, Hradec Kralove 50005, Czech Republic d Department of Environmental Toxicology, University of California, Meyer Hall, One Shields Avenue, Davis, CA 95616-8588, USA e Institute of Pharmacology, Faculty of Medicine and Dentistry, Palacky University, Hnevotinska 3, 775 15 Olomouc, Czech Republic h i g h l i g h t s • Food constituents may interact with drug metabolizing pathways. • AhR–CYP1A1 pathway is involved in drug metabolism and carcinogenesis. • We examined effects of 21 anthocyanins on AhR–CYP1A1 signaling. • Human hepatocytes and cell lines HepG2 and LS174T were used as the models. • Tested anthocyanins possess very low potential for food–drug interactions. a r t i c l e i n f o a b s t r a c t -

Phytochemical, Antifungal and Acute Toxicity Studies of Mitracarpus Scaber Zucc
CSJ 11(2): December, 2020 ISSN: 2276 – 707X Namadina et al. ChemSearch Journal 11(2): 73 – 81, December, 2020 Publication of Chemical Society of Nigeria, Kano Chapter Received: 15/11/2020 Accepted: 02/12/2020 http://www.ajol.info/index.php/csj Phytochemical, Antifungal and Acute Toxicity Studies of Mitracarpus scaber Zucc. Whole Plant Extracts 1*Namadina, M. M., 2Mukhtar, A.U., 2Musa, F. M., 3Sani, M. H., 4Haruna, S., 1Nuhu, Y. and 5Umar, A. M. 1Department of Plant Biology, Bayero University, Kano, Nigeria. 2Department of Microbiology, Kaduna State University 3Department of Plant sciences and Biotechnology, Nasarawa State University, Keffi 4Department of Forestry, Audu Bako Collage of Agriculture, Dambatta 5Department of Remedial and General Studies, Audu Bako Collage of Agriculture, Dambatta *Correspondence Email: [email protected]; [email protected] ABSTRACT Mitracarpus scaber have been reported in the treatment of various ailments such as ulcer, cancer, skin diseases etc. It is therefore important to investigate these plant parts to ascertain their therapeutic potentials. The Mitracarpus scaber whole plant was extracted with water and methanol, screened for their phytochemical properties and antifungal effects. The plant samples were also investigated for alkaloid, flavonoids, saponins, tannins and phenolic contents using quantitative techniques. The antifungal activities of the plant samples were tested against Candida albicans, Trichophyton mentagrophytes, Microsporum auduounii and Aspergillus flavus. The Minimum Inhibitory Concentration (MIC) and Minimum Fungicidal Concentration (MFC) of the extracts were also determined. Flavonoid, steroid, triterpenes, tannins, carbohydrate, glycoside, phenols were detected in both extracts while anthraquinones was absent. Alkaloid was detected in the aqueous extract but absent in methanol extract. -

Escuela De Posgrado Maestría En Agronegocios
UNIVERSIDAD NACIONAL AGRARIA LA MOLINA ESCUELA DE POSGRADO MAESTRÍA EN AGRONEGOCIOS “ESTRATEGIAS DE MERCADO PARA FOMENTAR LA EXPORTACIÓN DE ARÁNDANO (Vaccinium spp.) DESDE LIMA A ESTADOS UNIDOS A PARTIR DEL 2017” Presentada por: JULIA ESTHER GAMARRA SOLÓRZANO TESIS PARA OPTAR EL GRADO DE MAGISTER SCIENTIAE EN AGRONEGOCIOS Lima - Perú 2016 UNIVERSIDAD NACIONAL AGRARIA LA MOLINA ESCUELA DE POSGRADO MAESTRÍA EN AGRONEGOCIOS “ESTRATEGIAS DE MERCADO PARA FOMENTAR LA EXPORTACIÓN DE ARÁNDANO (Vaccinium spp.) DESDE LIMA A ESTADOS UNIDOS A PARTIR DEL 2017” TESIS PARA OPTAR EL GRADO DE MAGISTER SCIENTIAE Presentada por: JULIA ESTHER GAMARRA SOLÓRZANO Sustentada y aprobada ante el siguiente jurado: Dr. Pedro Quiroz Quezada Mg.Sc. Luis Espinoza Villanueva PRESIDENTE PATROCINADOR Mto.Prof.CPC. Demetrio Tello Romero Dr. Ampelio Ferrando Perea MIEMBRO MIEMBRO DEDICATORIA La presente tesis la dedico a Dios, por haberme llenado de fuerza y optimismo para cumplir con este objetivo; a mi mamá Julia que ha sido un pilar fundamental en mi formación profesional, por su desprendimiento, sus consejos y por ser un gran ejemplo de perseverancia; a mi esposo Denis por su apoyo incondicional y estar siempre en los momentos difíciles brindándome su amor y su comprensión ; a mi hijo Thiago por su amor y ser el motor de mi vida ; a mi hermana Giuliana por ser siempre mi fortaleza ; a mi primo Tato por haberme siempre orientado a tomar mejores decisiones, a mi Negrita por ser mi segunda madre y motivarme siempre; y a toda mi familia por estar a mi lado en todo momento. Julia. AGRADECIMIENTO A la Universidad Nacional Agraria La Molina, por haberme brindado una formación académica de alto nivel y a los docentes de la Maestría en Agronegocios, por los conocimientos y enseñanzas impartidas durante mi formación post gradual.