Mechanisms of Arterial Remodeling: Lessons from Genetic Diseases
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Prevalence and Incidence of Rare Diseases: Bibliographic Data
Number 1 | January 2019 Prevalence and incidence of rare diseases: Bibliographic data Prevalence, incidence or number of published cases listed by diseases (in alphabetical order) www.orpha.net www.orphadata.org If a range of national data is available, the average is Methodology calculated to estimate the worldwide or European prevalence or incidence. When a range of data sources is available, the most Orphanet carries out a systematic survey of literature in recent data source that meets a certain number of quality order to estimate the prevalence and incidence of rare criteria is favoured (registries, meta-analyses, diseases. This study aims to collect new data regarding population-based studies, large cohorts studies). point prevalence, birth prevalence and incidence, and to update already published data according to new For congenital diseases, the prevalence is estimated, so scientific studies or other available data. that: Prevalence = birth prevalence x (patient life This data is presented in the following reports published expectancy/general population life expectancy). biannually: When only incidence data is documented, the prevalence is estimated when possible, so that : • Prevalence, incidence or number of published cases listed by diseases (in alphabetical order); Prevalence = incidence x disease mean duration. • Diseases listed by decreasing prevalence, incidence When neither prevalence nor incidence data is available, or number of published cases; which is the case for very rare diseases, the number of cases or families documented in the medical literature is Data collection provided. A number of different sources are used : Limitations of the study • Registries (RARECARE, EUROCAT, etc) ; The prevalence and incidence data presented in this report are only estimations and cannot be considered to • National/international health institutes and agencies be absolutely correct. -

Orphanet Report Series Rare Diseases Collection
Marche des Maladies Rares – Alliance Maladies Rares Orphanet Report Series Rare Diseases collection DecemberOctober 2013 2009 List of rare diseases and synonyms Listed in alphabetical order www.orpha.net 20102206 Rare diseases listed in alphabetical order ORPHA ORPHA ORPHA Disease name Disease name Disease name Number Number Number 289157 1-alpha-hydroxylase deficiency 309127 3-hydroxyacyl-CoA dehydrogenase 228384 5q14.3 microdeletion syndrome deficiency 293948 1p21.3 microdeletion syndrome 314655 5q31.3 microdeletion syndrome 939 3-hydroxyisobutyric aciduria 1606 1p36 deletion syndrome 228415 5q35 microduplication syndrome 2616 3M syndrome 250989 1q21.1 microdeletion syndrome 96125 6p subtelomeric deletion syndrome 2616 3-M syndrome 250994 1q21.1 microduplication syndrome 251046 6p22 microdeletion syndrome 293843 3MC syndrome 250999 1q41q42 microdeletion syndrome 96125 6p25 microdeletion syndrome 6 3-methylcrotonylglycinuria 250999 1q41-q42 microdeletion syndrome 99135 6-phosphogluconate dehydrogenase 67046 3-methylglutaconic aciduria type 1 deficiency 238769 1q44 microdeletion syndrome 111 3-methylglutaconic aciduria type 2 13 6-pyruvoyl-tetrahydropterin synthase 976 2,8 dihydroxyadenine urolithiasis deficiency 67047 3-methylglutaconic aciduria type 3 869 2A syndrome 75857 6q terminal deletion 67048 3-methylglutaconic aciduria type 4 79154 2-aminoadipic 2-oxoadipic aciduria 171829 6q16 deletion syndrome 66634 3-methylglutaconic aciduria type 5 19 2-hydroxyglutaric acidemia 251056 6q25 microdeletion syndrome 352328 3-methylglutaconic -

Mackenzie's Mission Gene & Condition List
Mackenzie’s Mission Gene & Condition List What conditions are being screened for in Mackenzie’s Mission? Genetic carrier screening offered through this research study has been carefully developed. It is focused on providing people with information about their chance of having children with a severe genetic condition occurring in childhood. The screening is designed to provide genetic information that is relevant and useful, and to minimise uncertain and unclear information. How the conditions and genes are selected The Mackenzie’s Mission reproductive genetic carrier screen currently includes approximately 1300 genes which are associated with about 750 conditions. The reason there are fewer conditions than genes is that some genetic conditions can be caused by changes in more than one gene. The gene list is reviewed regularly. To select the conditions and genes to be screened, a committee comprised of experts in genetics and screening was established including: clinical geneticists, genetic scientists, a genetic pathologist, genetic counsellors, an ethicist and a parent of a child with a genetic condition. The following criteria were developed and are used to select the genes to be included: • Screening the gene is technically possible using currently available technology • The gene is known to cause a genetic condition • The condition affects people in childhood • The condition has a serious impact on a person’s quality of life and/or is life-limiting o For many of the conditions there is no treatment or the treatment is very burdensome for the child and their family. For some conditions very early diagnosis and treatment can make a difference for the child. -

Tracheobronchial Stenosis in Keutel Syndrome
C O R R E S P O N D E N C E Tracheobronchial Stenosis in Keutel Syndrome Keutel syndrome is characterized by brachytelephalangism, abnormal cartilage calcification, peripheral pulmonary stenoses, and midfacial hypoplasia. We report the first case from East Asia in an 8-month-old boy who had the typical craniofacial appearance characterized by midfacial hypoplasia with a broad depressed nasal bridge (Fig. 1). The distal phalanges of fingers were thickened. Auscultation FIG.1 Midface hypoplasia is present with a depressed nasal revealed a grade 2-3/6 systolic murmur over heart, bridge and small nose. pronounced in the second and third intercostal space, and an inspiratory and expiratory stridor and wheezing over both lungs. Chest radiograph and computed tomography alternative to surgical resection. Endoscopy has been showed tracheobronchial cartilage calcification and suggested as the first choice for simple stenosis, and tracheobronchial stenosis, confirmed on bronchoscopy. success rate of 96% has been reported. So far, this Echocardiography revealed peripheral pulmonary approach has rarely been used in children. Our patient stenosis. accepted bronchoscopic cryotherapy and balloon dilatation four times, and the diameter of the subglottic Keutel syndrome is a rare autosomal recessive laryngeal stenosis was expanded from 3 mm to 4.5 mm. disease, with 27 reported cases from 19 families in The clinical symptoms improved after endoscopy, but he several countries; mostly from the Middle East. All of died of lung reinfection three weeks after discharge from them showed tracheobronchial calcification, and five of our hospital. them had stenosis of the tracheobronchial tree [1,2]. Our LI-FENG SUN AND XING CHEN, patient is the fifth patient with tracheobronchial stenosis, Department of Pediatrics, Provincial Hospital Affiliated to which should be emphasized as another remarkable Shandong University, Jinan, 250021, China. -

(12) Patent Application Publication (10) Pub. No.: US 2010/0210567 A1 Bevec (43) Pub
US 2010O2.10567A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2010/0210567 A1 Bevec (43) Pub. Date: Aug. 19, 2010 (54) USE OF ATUFTSINASATHERAPEUTIC Publication Classification AGENT (51) Int. Cl. A638/07 (2006.01) (76) Inventor: Dorian Bevec, Germering (DE) C07K 5/103 (2006.01) A6IP35/00 (2006.01) Correspondence Address: A6IPL/I6 (2006.01) WINSTEAD PC A6IP3L/20 (2006.01) i. 2O1 US (52) U.S. Cl. ........................................... 514/18: 530/330 9 (US) (57) ABSTRACT (21) Appl. No.: 12/677,311 The present invention is directed to the use of the peptide compound Thr-Lys-Pro-Arg-OH as a therapeutic agent for (22) PCT Filed: Sep. 9, 2008 the prophylaxis and/or treatment of cancer, autoimmune dis eases, fibrotic diseases, inflammatory diseases, neurodegen (86). PCT No.: PCT/EP2008/007470 erative diseases, infectious diseases, lung diseases, heart and vascular diseases and metabolic diseases. Moreover the S371 (c)(1), present invention relates to pharmaceutical compositions (2), (4) Date: Mar. 10, 2010 preferably inform of a lyophilisate or liquid buffersolution or artificial mother milk formulation or mother milk substitute (30) Foreign Application Priority Data containing the peptide Thr-Lys-Pro-Arg-OH optionally together with at least one pharmaceutically acceptable car Sep. 11, 2007 (EP) .................................. O7017754.8 rier, cryoprotectant, lyoprotectant, excipient and/or diluent. US 2010/0210567 A1 Aug. 19, 2010 USE OF ATUFTSNASATHERAPEUTIC ment of Hepatitis BVirus infection, diseases caused by Hepa AGENT titis B Virus infection, acute hepatitis, chronic hepatitis, full minant liver failure, liver cirrhosis, cancer associated with Hepatitis B Virus infection. 0001. The present invention is directed to the use of the Cancer, Tumors, Proliferative Diseases, Malignancies and peptide compound Thr-Lys-Pro-Arg-OH (Tuftsin) as a thera their Metastases peutic agent for the prophylaxis and/or treatment of cancer, 0008. -

Discover Dysplasias Gene Panel
Discover Dysplasias Gene Panel Discover Dysplasias tests 109 genes associated with skeletal dysplasias. This list is gathered from various sources, is not designed to be comprehensive, and is provided for reference only. This list is not medical advice and should not be used to make any diagnosis. Refer to lab reports in connection with potential diagnoses. Some genes below may also be associated with non-skeletal dysplasia disorders; those non-skeletal dysplasia disorders are not included on this list. Skeletal Disorders Tested Gene Condition(s) Inheritance ACP5 Spondyloenchondrodysplasia with immune dysregulation (SED) AR ADAMTS10 Weill-Marchesani syndrome (WMS) AR AGPS Rhizomelic chondrodysplasia punctata type 3 (RCDP) AR ALPL Hypophosphatasia AD/AR ANKH Craniometaphyseal dysplasia (CMD) AD Mucopolysaccharidosis type VI (MPS VI), also known as Maroteaux-Lamy ARSB syndrome AR ARSE Chondrodysplasia punctata XLR Spondyloepimetaphyseal dysplasia with joint laxity type 1 (SEMDJL1) B3GALT6 Ehlers-Danlos syndrome progeroid type 2 (EDSP2) AR Multiple joint dislocations, short stature and craniofacial dysmorphism with B3GAT3 or without congenital heart defects (JDSCD) AR Spondyloepimetaphyseal dysplasia (SEMD) Thoracic aortic aneurysm and dissection (TADD), with or without additional BGN features, also known as Meester-Loeys syndrome XL Short stature, facial dysmorphism, and skeletal anomalies with or without BMP2 cardiac anomalies AD Acromesomelic dysplasia AR Brachydactyly type A2 AD BMPR1B Brachydactyly type A1 AD Desbuquois dysplasia CANT1 Multiple epiphyseal dysplasia (MED) AR CDC45 Meier-Gorlin syndrome AR This list is gathered from various sources, is not designed to be comprehensive, and is provided for reference only. This list is not medical advice and should not be used to make any diagnosis. -
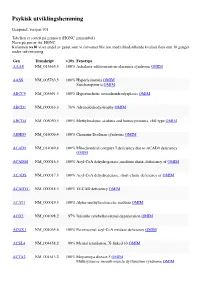
Psykisk Utviklingshemming
Psykisk utviklingshemming Genpanel, versjon v01 Tabellen er sortert på gennavn (HGNC gensymbol) Navn på gen er iht. HGNC Kolonnen >x10 viser andel av genet som vi forventer blir lest med tilfredstillende kvalitet flere enn 10 ganger under sekvensering Gen Transkript >10x Fenotype AAAS NM_015665.5 100% Achalasia-addisonianism-alacrimia syndrome OMIM AASS NM_005763.3 100% Hyperlysinemia OMIM Saccharopinuria OMIM ABCC9 NM_005691.3 100% Hypertrichotic osteochondrodysplasia OMIM ABCD1 NM_000033.3 76% Adrenoleukodystrophy OMIM ABCD4 NM_005050.3 100% Methylmalonic aciduria and homocystinuria, cblJ type OMIM ABHD5 NM_016006.4 100% Chanarin-Dorfman syndrome OMIM ACAD9 NM_014049.4 100% Mitochondrial complex I deficiency due to ACAD9 deficiency OMIM ACADM NM_000016.5 100% Acyl-CoA dehydrogenase, medium chain, deficiency of OMIM ACADS NM_000017.3 100% Acyl-CoA dehydrogenase, short-chain, deficiency of OMIM ACADVL NM_000018.3 100% VLCAD deficiency OMIM ACAT1 NM_000019.3 100% Alpha-methylacetoacetic aciduria OMIM ACO2 NM_001098.2 97% Infantile cerebellar-retinal degeneration OMIM ACOX1 NM_004035.6 100% Peroxisomal acyl-CoA oxidase deficiency OMIM ACSL4 NM_004458.2 99% Mental retardation, X-linked 63 OMIM ACTA2 NM_001613.2 100% Moyamoya disease 5 OMIM Multisystemic smooth muscle dysfunction syndrome OMIM Gen Transkript >10x Fenotype ACTB NM_001101.3 100% ?Dystonia, juvenile-onset OMIM Baraitser-Winter syndrome 1 OMIM ACTG1 NM_001614.3 100% Baraitser-Winter syndrome 2 OMIM Deafness, autosomal dominant 20/26 OMIM ACVR1 NM_001105.4 100% Fibrodysplasia ossificans -

Download CGT Exome V2.0
CGT Exome version 2. -

Utviklingsavvik V02
2/1/2021 Utviklingsavvik v02 Avdeling for medisinsk genetikk Utviklingsavvik Genpanel, versjon v02 * Enkelte genomiske regioner har lav eller ingen sekvensdekning ved eksomsekvensering. Dette skyldes at de har stor likhet med andre områder i genomet, slik at spesifikk gjenkjennelse av disse områdene og påvisning av varianter i disse områdene, blir vanskelig og upålitelig. Disse genetiske regionene har vi identifisert ved å benytte USCS segmental duplication hvor områder større enn 1 kb og ≥90% likhet med andre regioner i genomet, gjenkjennes (https://genome.ucsc.edu). For noen gener ligger alle ekson i områder med segmentale duplikasjoner: ACTB, ACTG1, ASNS, ATAD3A, CA5A, CFC1, CLCNKB, CYCS, DDX11, GBA, GJA1, MSTO1, PIGC, RBM8A, RPL15, SBDS, SDHA, SHOX, SLC6A8 Vi gjør oppmerksom på at ved identifiseringav ekson oppstrøms for startkodon kan eksonnummereringen endres uten at transkript ID endres. Avdelingens websider har en full oversikt over områder som er affisert av segmentale duplikasjoner. ** Transkriptets kodende ekson. Ekson Gen Gen affisert (HGNC (HGNC Transkript Ekson** Fenotype av symbol) ID) segdup* AAAS 13666 NM_015665.6 1-16 Achalasia-addisonianism-alacrimia syndrome, 231550 AARS 20 NM_001605.2 2-21 Epileptic encephalopathy, early infantile, 29 616339 AARS2 21022 NM_020745.4 1-22 Combined oxidative phosphorylation deficiency 8, 614096 AASS 17366 NM_005763.4 2-24 Hyperlysinaemia (Disorders of histidine, tryptophan or lysine metabolism) ABAT 23 NM_020686.6 2-16 GABA transaminase deficiency (Disorders of neurotransmitter metabolism, gamma-aminobutyrate) -

Whole Exome Sequencing Gene Package Multiple Congenital Anomaly, Version 8.1, 31-1-2020
Whole Exome Sequencing Gene package Multiple congenital anomaly, version 8.1, 31-1-2020 Technical information DNA was enriched using Agilent SureSelect DNA + SureSelect OneSeq 300kb CNV Backbone + Human All Exon V7 capture and paired-end sequenced on the Illumina platform (outsourced). The aim is to obtain 10 Giga base pairs per exome with a mapped fraction of 0.99. The average coverage of the exome is ~50x. Duplicate and non-unique reads are excluded. Data are demultiplexed with bcl2fastq Conversion Software from Illumina. Reads are mapped to the genome using the BWA-MEM algorithm (reference: http://bio-bwa.sourceforge.net/). Variant detection is performed by the Genome Analysis Toolkit HaplotypeCaller (reference: http://www.broadinstitute.org/gatk/). The detected variants are filtered and annotated with Cartagenia software and classified with Alamut Visual. It is not excluded that pathogenic mutations are being missed using this technology. At this moment, there is not enough information about the sensitivity of this technique with respect to the detection of deletions and duplications of more than 5 nucleotides and of somatic mosaic mutations (all types of sequence changes). HGNC approved Phenotype description including OMIM phenotype ID(s) OMIM median depth % covered % covered % covered gene symbol gene ID >10x >20x >30x A4GALT [Blood group, P1Pk system, P(2) phenotype], 111400 [Blood group, P1Pk system, p phenotype], 111400 NOR poly607922 146 100 100 99 AAAS Achalasia-addisonianism-alacrimia syndrome, 231550 605378 102 100 100 100 -

Genomeposter2009.Pdf
Fold HumanSelected Genome Genes, Traits, and Landmarks Disorders www.ornl.gov/hgmis/posters/chromosome genomics.energy. -

Encephalopathies with Intracranial
Tonduti et al. Orphanet Journal of Rare Diseases (2018) 13:135 https://doi.org/10.1186/s13023-018-0854-y RESEARCH Open Access Encephalopathies with intracranial calcification in children: clinical and genetic characterization Davide Tonduti1,2* , Celeste Panteghini3, Anna Pichiecchio4, Alice Decio5,6, Miryam Carecchio1,3,7, Chiara Reale3, Isabella Moroni1, Nardo Nardocci1, Jaume Campistol8, Angela Garcia-Cazorla8, Belen Perez Duenas8, Cerebral Calcification International Study Group, Luisa Chiapparini9, Barbara Garavaglia3 and Simona Orcesi5 Abstract Background: We present a group of patients affected by a paediatric onset genetic encephalopathy with cerebral calcification of unknown aetiology studied with Next Generation Sequencing (NGS) genetic analyses. Methods: We collected all clinical and radiological data. DNA samples were tested by means of a customized gene panel including fifty-nine genes associated with known genetic diseases with cerebral calcification. Results: We collected a series of fifty patients. All patients displayed complex and heterogeneous phenotypes mostly including developmental delay and pyramidal signs and less frequently movement disorder and epilepsy. Signs of cerebellar and peripheral nervous system involvement were occasionally present. The most frequent MRI abnormality, beside calcification, was the presence of white matter alterations; calcification was localized in basal ganglia and cerebral white matter in the majority of cases. Sixteen out of fifty patients tested positive for mutations in one of the fifty-nine genes analyzed. In fourteen cases the analyses led to a definite genetic diagnosis while results were controversial in the remaining two. Conclusions: Genetic encephalopathies with cerebral calcification are usually associated to complex phenotypes. In our series, a molecular diagnosis was achieved in 32% of cases, suggesting that the molecular bases of a large number of disorders are still to be elucidated.