Utviklingsavvik V02
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
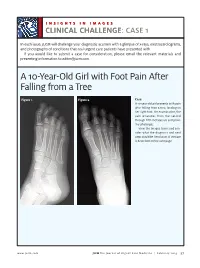
A 10-Year-Old Girl with Foot Pain After Falling from a Tree
INSIGHTS IN IMAGES CLINICAL CHALLENGECHALLENGE: CASE 1 In each issue, JUCM will challenge your diagnostic acumen with a glimpse of x-rays, electrocardiograms, and photographs of conditions that real urgent care patients have presented with. If you would like to submit a case for consideration, please email the relevant materials and presenting information to [email protected]. A 10-Year-Old Girl with Foot Pain After Falling from a Tree Figure 1. Figure 2. Case A 10-year-old girl presents with pain after falling from a tree, landing on her right foot. On examination, the pain emanates from the second through fifth metatarsals and proxi- mal phalanges. View the images taken and con- sider what the diagnosis and next steps would be. Resolution of the case is described on the next page. www.jucm.com JUCM The Journal of Urgent Care Medicine | February 2019 37 INSIGHTS IN IMAGES: CLINICAL CHALLENGE THE RESOLUTION Figure 1. Mediastinal air Figure 1. Differential Diagnosis Pearls for Urgent Care Management and Ⅲ Fracture of the distal fourth metatarsal Considerations for Transfer Ⅲ Plantar plate disruption Ⅲ Emergent transfer should be considered with associated neu- Ⅲ Sesamoiditis rologic deficit, compartment syndrome, open fracture, or vas- Ⅲ Turf toe cular compromise Ⅲ Referral to an orthopedist is warranted in the case of an in- Diagnosis tra-articular fracture, or with Lisfranc ligament injury or ten- Angulation of the distal fourth metatarsal metaphyseal cortex derness over the Lisfranc ligament and hairline lucency consistent with fracture. Acknowledgment: Images courtesy of Teleradiology Associates. Learnings/What to Look for Ⅲ Proximal metatarsal fractures are most often caused by crush- ing or direct blows Ⅲ In athletes, an axial load placed on a plantar-flexed foot should raise suspicion of a Lisfranc injury 38 JUCM The Journal of Urgent Care Medicine | February 2019 www.jucm.com INSIGHTS IN IMAGES CLINICAL CHALLENGE: CASE 2 A 55-Year-Old Man with 3 Hours of Epigastric Pain 55 years PR 249 QRSD 90 QT 471 QTc 425 AXES P 64 QRS -35 T 30 Figure 1. -

Centronuclear Myopathies Under Attack: a Plethora of Therapeutic Targets Hichem Tasfaout, Belinda Cowling, Jocelyn Laporte
CORE Metadata, citation and similar papers at core.ac.uk Provided by Archive Ouverte en Sciences de l'Information et de la Communication Centronuclear myopathies under attack: A plethora of therapeutic targets Hichem Tasfaout, Belinda Cowling, Jocelyn Laporte To cite this version: Hichem Tasfaout, Belinda Cowling, Jocelyn Laporte. Centronuclear myopathies under attack: A plethora of therapeutic targets. Journal of Neuromuscular Diseases, IOS Press, 2018, 5, pp.387 - 406. 10.3233/JND-180309. hal-02438924 HAL Id: hal-02438924 https://hal.archives-ouvertes.fr/hal-02438924 Submitted on 14 Jan 2020 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Journal of Neuromuscular Diseases 5 (2018) 387–406 387 DOI 10.3233/JND-180309 IOS Press Review Centronuclear myopathies under attack: A plethora of therapeutic targets Hichem Tasfaouta,b,c,d, Belinda S. Cowlinga,b,c,d,1 and Jocelyn Laportea,b,c,d,1,∗ aDepartment of Translational Medicine and Neurogenetics, Institut de G´en´etique et de Biologie Mol´eculaire et Cellulaire (IGBMC), Illkirch, France bInstitut National de la Sant´eetdelaRechercheM´edicale (INSERM), U1258, Illkirch, France cCentre National de la Recherche Scientifique (CNRS), UMR7104, Illkirch, France dUniversit´e de Strasbourg, Illkirch, France Abstract. -

Moderate the MAOA-L Allele Expression with CRISPR/Cas9 System
Preprints (www.preprints.org) | NOT PEER-REVIEWED | Posted: 23 April 2018 doi:10.20944/preprints201804.0275.v1 1 Review 2 Moderate the MAOA-L Allele Expression with CRISPR/Cas9 System 3 Martin L. Nelwan 4 Department of Animal Science – Other 5 Nelwan Institution for Human Resource Development 6 Jl. A. Yani No. 24 7 Palu, Sulawesi Tengah, Indonesia 8 Email: [email protected] 9 Abstract: Antisocial behavior is a behavior disorder inherited according to the inheritance of X-linked 10 chromosome. Mutations in the MAOA gene can cause different behaviors in humans. These can comprise 11 violent behavior or antisocial behavior. Low MAOA (MAOA-L) allele activity can cause antisocial 12 behavior in both healthy and unhealthy people. Antisocial from healthy males can originate from 13 maltreatment during childhood. There are no drugs for the treatment of antisocial behavior permanently 14 at this time. MAOA inhibitor can reverse antisocial behavior in animal models. To cure antisocial 15 behavior in the future, the CRISPR/Cas9 system in combination with iPSCs or ssODN methods for 16 instance can be used. This system has succeeded to correct erroneous segments in the F8 gene and F9 17 gene. Both genes occupy the X chromosome. The MAOA gene also occupies the X chromosome. It seems 18 that CRISPR/Cas9 system may be a beneficial tool to edit erroneous segments in the MAOA gene to treat 19 antisocial behavior. 20 Keywords: advanced therapy, aggressive, antisocial, behavior, MAOA. 21 22 1. Introduction 23 Antisocial behavior is a hereditary disorder inherited through an X-linked recessive inheritance 24 pattern. -

Genetic Mechanisms of Disease in Children: a New Look
Genetic Mechanisms of Disease in Children: A New Look Laurie Demmer, MD Tufts Medical Center and the Floating Hospital for Children Boston, MA Traditionally genetic disorders have been linked to the ‘one gene-one protein-one disease’ hypothesis. However recent advances in the field of molecular biology and biotechnology have afforded us the opportunity to greatly expand our knowledge of genetics, and we now know that the mechanisms of inherited disorders are often significantly more complex, and consequently, much more intriguing, than originally thought. Classical mendelian disorders with relatively simple genetic mechanisms do exist, but turn out to be far more rare than originally thought. All patients with sickle cell disease for example, carry the same A-to-T point mutation in the sixth codon of the beta globin gene. This results in a glutamate to valine substitution which changes the shape and the function of the globin molecule in a predictable way. Similarly, all patients with achondroplasia have a single base pair substitution at nucleotide #1138 of the FGFR3 gene. On the other hand, another common inherited disorder, cystic fibrosis, is known to result from changes in a specific transmembrane receptor (CFTR), but over 1000 different disease-causing mutations have been reported in this single gene. Since most commercial labs only test for between 23-100 different mutations, interpreting CFTR mutation testing is significantly complicated by the known risk of false negative results. Many examples of complex, or non-Mendelian, inheritance are now known to exist and include disorders of trinucleotide repeats, errors in imprinting, and gene dosage effects. -

Affected Female Carriers of MTM1 Mutations Display a Wide Spectrum
Acta Neuropathol DOI 10.1007/s00401-017-1748-0 ORIGINAL PAPER Afected female carriers of MTM1 mutations display a wide spectrum of clinical and pathological involvement: delineating diagnostic clues Valérie Biancalana1,2,3,4,5 · Sophie Scheidecker1 · Marguerite Miguet1 · Annie Laquerrière6 · Norma B. Romero7,8 · Tanya Stojkovic8 · Osorio Abath Neto9 · Sandra Mercier10,11,12 · Nicol Voermans13 · Laura Tanner14 · Curtis Rogers15 · Elisabeth Ollagnon‑Roman16 · Helen Roper17 · Célia Boutte18 · Shay Ben‑Shachar19 · Xavière Lornage2,3,4,5 · Nasim Vasli2,3,4,5 · Elise Schaefer20 · Pascal Laforet21 · Jean Pouget22 · Alexandre Moerman23 · Laurent Pasquier24 · Pascale Marcorelle25,26 · Armelle Magot12 · Benno Küsters27 · Nathalie Streichenberger28 · Christine Tranchant29 · Nicolas Dondaine1 · Raphael Schneider2,3,4,5,30 · Claire Gasnier1 · Nadège Calmels1 · Valérie Kremer31 · Karine Nguyen32 · Julie Perrier12 · Erik Jan Kamsteeg33 · Pierre Carlier34 · Robert‑Yves Carlier35 · Julie Thompson30 · Anne Boland36 · Jean‑François Deleuze36 · Michel Fardeau7,8 · Edmar Zanoteli9 · Bruno Eymard21 · Jocelyn Laporte2,3,4,5 Received: 9 May 2017 / Revised: 24 June 2017 / Accepted: 2 July 2017 © Springer-Verlag GmbH Germany 2017 Abstract X-linked myotubular myopathy (XLMTM), a females and to delineate diagnostic clues, we character- severe congenital myopathy, is caused by mutations in the ized 17 new unrelated afected females and performed a MTM1 gene located on the X chromosome. A majority of detailed comparison with previously reported cases at the afected males die in the early postnatal period, whereas clinical, muscle imaging, histological, ultrastructural and female carriers are believed to be usually asymptomatic. molecular levels. Taken together, the analysis of this large Nevertheless, several afected females have been reported. cohort of 43 cases highlights a wide spectrum of clini- To assess the phenotypic and pathological spectra of carrier cal severity ranging from severe neonatal and generalized weakness, similar to XLMTM male, to milder adult forms. -

Prestin, a Cochlear Motor Protein, Is Defective in Non-Syndromic Hearing Loss
Human Molecular Genetics, 2003, Vol. 12, No. 10 1155–1162 DOI: 10.1093/hmg/ddg127 Prestin, a cochlear motor protein, is defective in non-syndromic hearing loss Xue Zhong Liu1,*, Xiao Mei Ouyang1, Xia Juan Xia2, Jing Zheng3, Arti Pandya2, Fang Li1, Li Lin Du1, Katherine O. Welch4, Christine Petit5, Richard J.H. Smith6, Bradley T. Webb2, Denise Yan1, Kathleen S. Arnos4, David Corey7, Peter Dallos3, Walter E. Nance2 and Zheng Yi Chen8 1Department of Otolaryngology, University of Miami, Miami, FL 33101, USA, 2Department of Human Genetics, Medical College of Virginia, Virginia Commonwealth University, Richmond, VA 23298-0033, USA, 3Department of Communication Sciences and Disorders, Auditory Physiology Laboratory (The Hugh Knowles Center), Northwestern University, Evanston, IL, USA, 4Department of Biology, Gallaudet University, Washington, DC 20002, USA, 5Unite´ de Ge´ne´tique des De´ficits Sensoriels, CNRS URA 1968, Institut Pasteur, Paris, France, 6Department of Otolaryngology University of Iowa, Iowa City, IA 52242, USA, 7Neurobiology Department, Harvard Medical School and Howard Hughes Medical Institute, Boston, MA, USA and 8Department of Neurology, Massachusetts General Hospital and Neurology Department, Harvard Medical School Boston, MA 02114, USA Received January 14, 2003; Revised and Accepted March 14, 2003 Prestin, a membrane protein that is highly and almost exclusively expressed in the outer hair cells (OHCs) of the cochlea, is a motor protein which senses membrane potential and drives rapid length changes in OHCs. Surprisingly, prestin is a member of a gene family, solute carrier (SLC) family 26, that encodes anion transporters and related proteins. Of nine known human genes in this family, three (SLC26A2, SLC26A3 and SLC26A4 ) are associated with different human hereditary diseases. -

2016 IES Annual Meeting Final Programme
ROYAL ACADEMY OF MEDICINE IN IRELAND IRISH JOURNAL OF MEDICAL SCIENCE Irish Endocrine Society 40th Annual Meeting 14th and 15th October 2016 Stormont Hotel, Belfast Local Organiser: Doctor Hamish Courtney, REVISEDRoyal Victoria Hospital, PROOF Belfast Irish Journal of Medical Science Volume XXX Supplement X DOI 10.1007/s11845-016-1482-y 123 123 Journal : Large 11845 Dispatch : 17-8-2016 Pages : 57 Article No. : 1482 h LE h TYPESET MS Code : 1482 h44CP h DISK Ir J Med Sci Disclosure statement This supplement is paid for by the Irish Endocrine Society. However the meeting costs are supported by the following commercial sponsors: Abbott Amgen Astra Zeneca Besins Healthcare BMS Boehringer Ingleheim Consilient Ipsen Janssen-Cilag Kyowa Kirin Lilly Menarini Merck Serono MSD Novartis Novo Nordisk Pfizer Sanofi REVISED PROOF 123 Journal : Large 11845 Dispatch : 17-8-2016 Pages : 57 Article No. : 1482 h LE h TYPESET MS Code : 1482 h44CP h DISK Ir J Med Sci Novo Lecture Nordisk Lecture 1976 D.K. O’Donovan 1977 S. Bloom 1978 J.H.S. Robertson 1979 A.G. Cudworth 1980 D.A.D. Montgomery 1981 Peter Watkins 1982 G. Joplin 1983 D.R. London 1984 A.X. Bertagna 1985 Malcolm Nattrass Laurence Kennedy 1986 Brian Frier JB Ferriss 1987 Maurice Scanlon TJ McKenna 1988 D.A. Heath AB Atkinson 1989 J. Ward GH Tomkin 1990 R. Volpe KD Buchanan 1991 Michael Besser PPA Smyth 1992 R.V. Ragontte DH Hadden 1993 Bruce Weintraub David Powell 1994 Oscar Croffard Patrick Bell 1995 Robert Lindsay Brian Sheridan 1996 C.R.W. Edwards Rosemary Freaney 1997 Stephanie Amiel David McCance 1998 Robert Turner Randle Hayes 1999 Ian Hay Sean K Cunningham 2000 Stephen O’Rahilly Michael Cullen 2001 Andre Lacroix Daphne Owens 2002 J. -

Supplementary Table 3 Complete List of RNA-Sequencing Analysis of Gene Expression Changed by ≥ Tenfold Between Xenograft and Cells Cultured in 10%O2
Supplementary Table 3 Complete list of RNA-Sequencing analysis of gene expression changed by ≥ tenfold between xenograft and cells cultured in 10%O2 Expr Log2 Ratio Symbol Entrez Gene Name (culture/xenograft) -7.182 PGM5 phosphoglucomutase 5 -6.883 GPBAR1 G protein-coupled bile acid receptor 1 -6.683 CPVL carboxypeptidase, vitellogenic like -6.398 MTMR9LP myotubularin related protein 9-like, pseudogene -6.131 SCN7A sodium voltage-gated channel alpha subunit 7 -6.115 POPDC2 popeye domain containing 2 -6.014 LGI1 leucine rich glioma inactivated 1 -5.86 SCN1A sodium voltage-gated channel alpha subunit 1 -5.713 C6 complement C6 -5.365 ANGPTL1 angiopoietin like 1 -5.327 TNN tenascin N -5.228 DHRS2 dehydrogenase/reductase 2 leucine rich repeat and fibronectin type III domain -5.115 LRFN2 containing 2 -5.076 FOXO6 forkhead box O6 -5.035 ETNPPL ethanolamine-phosphate phospho-lyase -4.993 MYO15A myosin XVA -4.972 IGF1 insulin like growth factor 1 -4.956 DLG2 discs large MAGUK scaffold protein 2 -4.86 SCML4 sex comb on midleg like 4 (Drosophila) Src homology 2 domain containing transforming -4.816 SHD protein D -4.764 PLP1 proteolipid protein 1 -4.764 TSPAN32 tetraspanin 32 -4.713 N4BP3 NEDD4 binding protein 3 -4.705 MYOC myocilin -4.646 CLEC3B C-type lectin domain family 3 member B -4.646 C7 complement C7 -4.62 TGM2 transglutaminase 2 -4.562 COL9A1 collagen type IX alpha 1 chain -4.55 SOSTDC1 sclerostin domain containing 1 -4.55 OGN osteoglycin -4.505 DAPL1 death associated protein like 1 -4.491 C10orf105 chromosome 10 open reading frame 105 -4.491 -

Preconception Carrier Screening by Genome Sequencing: Results from the Clinical Laboratory
The American Journal of Human Genetics, Volume 102 Supplemental Data Preconception Carrier Screening by Genome Sequencing: Results from the Clinical Laboratory Sumit Punj, Yassmine Akkari, Jennifer Huang, Fei Yang, Allison Creason, Christine Pak, Amiee Potter, Michael O. Dorschner, Deborah A. Nickerson, Peggy D. Robertson, Gail P. Jarvik, Laura M. Amendola, Jennifer Schleit, Dana Kostiner Simpson, Alan F. Rope, Jacob Reiss, Tia Kauffman, Marian J. Gilmore, Patricia Himes, Benjamin Wilfond, Katrina A.B. Goddard, and C. Sue Richards Supplemental Note: Clinical Report Carrier Results: Four Known Pathogenic Variants Detected. Gene Inheritance Disease Prevalence Variant Classification Pendred Syndrome/ Non- syndromic Autosomal Hearing Loss A c.1246A>C, SLC26A4 1/500 Pathogenic Recessive DFNB4 with (p.Thr416Pro) enlarged vestibular aqueduct Autosomal Spastic ++ c.1045G>A, SPG7 2-6/100,000 Pathogenic Recessive Paraplegia 7 (p.Gly349Ser) 3.7 Autosomal Alpha +++ -α HBA2 1-5/10,000 Pathogenic Recessive Thalassemia (α+- thalassemia) Autosomal Hereditary 1/200 – c.845G>A HFE Pathogenic Recessive Hemochromatosis 1/1000+ (p.Cys282Tyr) +: GeneReviews; ++: Genetics Home Reference; +++: orphan.net – varies with population; A- Generalized prevalence of all deafness and hearing loss Interpretation: A sample from this individual was referred to our laboratory for analysis of Next-Generation Genome Sequencing (NGS) and Sanger confirmation of variants identified in carrier screening for: (1) conditions with significantly shortened lifespan; (2) serious conditions; (3) mild conditions; (4) conditions with unpredictable outcomes: and (5) conditions that begin as adults. One known heterozygous missense variant, c.1246A>C (p.Thr416Pro) (NM_000441.1), was detected in exon 10 of the SLC26A4 gene of this individual by NGS. -

A SARS-Cov-2-Human Protein-Protein Interaction Map Reveals Drug Targets and Potential Drug-Repurposing
A SARS-CoV-2-Human Protein-Protein Interaction Map Reveals Drug Targets and Potential Drug-Repurposing Supplementary Information Supplementary Discussion All SARS-CoV-2 protein and gene functions described in the subnetwork appendices, including the text below and the text found in the individual bait subnetworks, are based on the functions of homologous genes from other coronavirus species. These are mainly from SARS-CoV and MERS-CoV, but when available and applicable other related viruses were used to provide insight into function. The SARS-CoV-2 proteins and genes listed here were designed and researched based on the gene alignments provided by Chan et. al. 1 2020 . Though we are reasonably sure the genes here are well annotated, we want to note that not every protein has been verified to be expressed or functional during SARS-CoV-2 infections, either in vitro or in vivo. In an effort to be as comprehensive and transparent as possible, we are reporting the sub-networks of these functionally unverified proteins along with the other SARS-CoV-2 proteins. In such cases, we have made notes within the text below, and on the corresponding subnetwork figures, and would advise that more caution be taken when examining these proteins and their molecular interactions. Due to practical limits in our sample preparation and data collection process, we were unable to generate data for proteins corresponding to Nsp3, Orf7b, and Nsp16. Therefore these three genes have been left out of the following literature review of the SARS-CoV-2 proteins and the protein-protein interactions (PPIs) identified in this study. -

Skeletal Dysplasias
Skeletal Dysplasias North Carolina Ultrasound Society Keisha L.B. Reddick, MD Wilmington Maternal Fetal Medicine Development of the Skeleton • 6 weeks – vertebrae • 7 weeks – skull • 8 wk – clavicle and mandible – Hyaline cartilage • Ossification – 7-12 wk – diaphysis appears – 12-16 wk metacarpals and metatarsals – 20+ wk pubis, calus, calcaneus • Visualization of epiphyseal ossification centers Epidemiology • Overall 9.1 per 1000 • Lethal 1.1 per 10,000 – Thanatophoric 1/40,000 – Osteogenesis Imperfecta 0.18 /10,000 – Campomelic 0.1 /0,000 – Achondrogenesis 0.1 /10,000 • Non-lethal – Achondroplasia 15 in 10,000 Most Common Skeletal Dysplasia • Thantophoric dysplasia 29% • Achondroplasia 15% • Osteogenesis imperfecta 14% • Achondrogenesis 9% • Campomelic dysplasia 2% Definition/Terms • Rhizomelia – proximal segment • Mezomelia –intermediate segment • Acromelia – distal segment • Micromelia – all segments • Campomelia – bowing of long bones • Preaxial – radial/thumb or tibial side • Postaxial – ulnar/little finger or fibular Long Bone Segments Counseling • Serial ultrasound • Genetic counseling • Genetic testing – Amniocentesis • Postnatal – Delivery center – Radiographs Assessment • Which segment is affected • Assessment of distal extremities • Any curvatures, fracture or clubbing noted • Are metaphyseal changes present • Hypoplastic or absent bones • Assessment of the spinal canal • Assessment of thorax. Skeletal Dysplasia Lethal Non-lethal • Thanatophoric • Achondroplasia • OI type II • OI type I, III, IV • Achondrogenesis • Hypochondroplasia -

Genes in Eyecare Geneseyedoc 3 W.M
Genes in Eyecare geneseyedoc 3 W.M. Lyle and T.D. Williams 15 Mar 04 This information has been gathered from several sources; however, the principal source is V. A. McKusick’s Mendelian Inheritance in Man on CD-ROM. Baltimore, Johns Hopkins University Press, 1998. Other sources include McKusick’s, Mendelian Inheritance in Man. Catalogs of Human Genes and Genetic Disorders. Baltimore. Johns Hopkins University Press 1998 (12th edition). http://www.ncbi.nlm.nih.gov/Omim See also S.P.Daiger, L.S. Sullivan, and B.J.F. Rossiter Ret Net http://www.sph.uth.tmc.edu/Retnet disease.htm/. Also E.I. Traboulsi’s, Genetic Diseases of the Eye, New York, Oxford University Press, 1998. And Genetics in Primary Eyecare and Clinical Medicine by M.R. Seashore and R.S.Wappner, Appleton and Lange 1996. M. Ridley’s book Genome published in 2000 by Perennial provides additional information. Ridley estimates that we have 60,000 to 80,000 genes. See also R.M. Henig’s book The Monk in the Garden: The Lost and Found Genius of Gregor Mendel, published by Houghton Mifflin in 2001 which tells about the Father of Genetics. The 3rd edition of F. H. Roy’s book Ocular Syndromes and Systemic Diseases published by Lippincott Williams & Wilkins in 2002 facilitates differential diagnosis. Additional information is provided in D. Pavan-Langston’s Manual of Ocular Diagnosis and Therapy (5th edition) published by Lippincott Williams & Wilkins in 2002. M.A. Foote wrote Basic Human Genetics for Medical Writers in the AMWA Journal 2002;17:7-17. A compilation such as this might suggest that one gene = one disease.