Megalencephaly and Macrocephaly
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
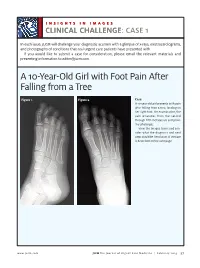
A 10-Year-Old Girl with Foot Pain After Falling from a Tree
INSIGHTS IN IMAGES CLINICAL CHALLENGECHALLENGE: CASE 1 In each issue, JUCM will challenge your diagnostic acumen with a glimpse of x-rays, electrocardiograms, and photographs of conditions that real urgent care patients have presented with. If you would like to submit a case for consideration, please email the relevant materials and presenting information to [email protected]. A 10-Year-Old Girl with Foot Pain After Falling from a Tree Figure 1. Figure 2. Case A 10-year-old girl presents with pain after falling from a tree, landing on her right foot. On examination, the pain emanates from the second through fifth metatarsals and proxi- mal phalanges. View the images taken and con- sider what the diagnosis and next steps would be. Resolution of the case is described on the next page. www.jucm.com JUCM The Journal of Urgent Care Medicine | February 2019 37 INSIGHTS IN IMAGES: CLINICAL CHALLENGE THE RESOLUTION Figure 1. Mediastinal air Figure 1. Differential Diagnosis Pearls for Urgent Care Management and Ⅲ Fracture of the distal fourth metatarsal Considerations for Transfer Ⅲ Plantar plate disruption Ⅲ Emergent transfer should be considered with associated neu- Ⅲ Sesamoiditis rologic deficit, compartment syndrome, open fracture, or vas- Ⅲ Turf toe cular compromise Ⅲ Referral to an orthopedist is warranted in the case of an in- Diagnosis tra-articular fracture, or with Lisfranc ligament injury or ten- Angulation of the distal fourth metatarsal metaphyseal cortex derness over the Lisfranc ligament and hairline lucency consistent with fracture. Acknowledgment: Images courtesy of Teleradiology Associates. Learnings/What to Look for Ⅲ Proximal metatarsal fractures are most often caused by crush- ing or direct blows Ⅲ In athletes, an axial load placed on a plantar-flexed foot should raise suspicion of a Lisfranc injury 38 JUCM The Journal of Urgent Care Medicine | February 2019 www.jucm.com INSIGHTS IN IMAGES CLINICAL CHALLENGE: CASE 2 A 55-Year-Old Man with 3 Hours of Epigastric Pain 55 years PR 249 QRSD 90 QT 471 QTc 425 AXES P 64 QRS -35 T 30 Figure 1. -

Genetic Mechanisms of Disease in Children: a New Look
Genetic Mechanisms of Disease in Children: A New Look Laurie Demmer, MD Tufts Medical Center and the Floating Hospital for Children Boston, MA Traditionally genetic disorders have been linked to the ‘one gene-one protein-one disease’ hypothesis. However recent advances in the field of molecular biology and biotechnology have afforded us the opportunity to greatly expand our knowledge of genetics, and we now know that the mechanisms of inherited disorders are often significantly more complex, and consequently, much more intriguing, than originally thought. Classical mendelian disorders with relatively simple genetic mechanisms do exist, but turn out to be far more rare than originally thought. All patients with sickle cell disease for example, carry the same A-to-T point mutation in the sixth codon of the beta globin gene. This results in a glutamate to valine substitution which changes the shape and the function of the globin molecule in a predictable way. Similarly, all patients with achondroplasia have a single base pair substitution at nucleotide #1138 of the FGFR3 gene. On the other hand, another common inherited disorder, cystic fibrosis, is known to result from changes in a specific transmembrane receptor (CFTR), but over 1000 different disease-causing mutations have been reported in this single gene. Since most commercial labs only test for between 23-100 different mutations, interpreting CFTR mutation testing is significantly complicated by the known risk of false negative results. Many examples of complex, or non-Mendelian, inheritance are now known to exist and include disorders of trinucleotide repeats, errors in imprinting, and gene dosage effects. -

Pfeiffer Syndrome Type II Discovered Perinatally
Diagnostic and Interventional Imaging (2012) 93, 785—789 CORE Metadata, citation and similar papers at core.ac.uk Provided by Elsevier - Publisher Connector LETTER / Musculoskeletal imaging Pfeiffer syndrome type II discovered perinatally: Report of an observation and review of the literature a,∗ a a a H. Ben Hamouda , Y. Tlili , S. Ghanmi , H. Soua , b c b a S. Jerbi , M.M. Souissi , H. Hamza , M.T. Sfar a Unité de néonatologie, service de pédiatrie, CHU Tahar Sfar, 5111 Mahdia, Tunisia b Service de radiologie, CHU Tahar Sfar, 5111 Mahdia, Tunisia c Service de gynéco-obstétrique, CHU Tahar Sfar, 5111 Mahdia, Tunisia Pfeiffer syndrome, described for the first time by Pfeiffer in 1964, is a rare hereditary KEYWORDS condition combining osteochondrodysplasia with craniosynostosis [1]. This syndrome is Pfeiffer syndrome; also called acrocephalosyndactyly type 5, which is divided into three sub-types. Type I Cloverleaf skull; is the classic Pfeiffer syndrome, with autosomal dominant transmission, often associated Craniosynostosis; with normal intelligence. Types II and III occur as sporadic cases in individuals who have Syndactyly; craniosynostosis with broad thumbs, broad big toes, ankylosis of the elbows and visceral Prenatal diagnosis abnormalities [2]. We report a case of Pfeiffer syndrome type II, discovered perinatally, which is distinguished from type III by the skull appearing like a cloverleaf, and we shall discuss the clinical, radiological and evolutive features and the advantage of prenatal diagnosis of this syndrome with a review of the literature. Observation The case involved a male premature baby born at 36 weeks of amenorrhoea with multiple deformities at birth. The parents were not blood-related and in good health who had two other boys and a girl with normal morphology. -

Segmental Overvekst Og Vaskulærmalformasjoner V02
2/1/2021 Segmental overvekst og vaskulærmalformasjoner v02 Avdeling for medisinsk genetikk Segmental overvekst og vaskulærmalformasjoner Genpanel, versjon v02 * Enkelte genomiske regioner har lav eller ingen sekvensdekning ved eksomsekvensering. Dette skyldes at de har stor likhet med andre områder i genomet, slik at spesifikk gjenkjennelse av disse områdene og påvisning av varianter i disse områdene, blir vanskelig og upålitelig. Disse genetiske regionene har vi identifisert ved å benytte USCS segmental duplication hvor områder større enn 1 kb og ≥90% likhet med andre regioner i genomet, gjenkjennes (https://genome.ucsc.edu). Vi gjør oppmerksom på at ved identifiseringav ekson oppstrøms for startkodon kan eksonnummereringen endres uten at transkript ID endres. Avdelingens websider har en full oversikt over områder som er affisert av segmentale duplikasjoner. ** Transkriptets kodende ekson. Ekson Gen Gen affisert (HGNC (HGNC Transkript Ekson** Fenotype av symbol) ID) segdup* ACVRL1 175 NM_000020.3 2-10 Telangiectasia, hereditary hemorrhagic, type 2 OMIM ADAMTS3 219 NM_014243.3 1-22 Hennekam lymphangiectasia- lymphedema syndrome 3 OMIM AKT1 391 NM_005163.2 2-14 Cowden syndrome 6 OMIM Proteus syndrome, somatic OMIM AKT2 392 NM_001626.6 2-14 Diabetes mellitus, type II OMIM Hypoinsulinemic hypoglycemia with hemihypertrophy OMIM AKT3 393 NM_005465.7 2-14 Megalencephaly-polymicrogyria- polydactyly-hydrocephalus syndrome 2 OMIM file:///data/SegOv_v02-web.html 1/7 2/1/2021 Segmental overvekst og vaskulærmalformasjoner v02 Ekson Gen Gen affisert (HGNC (HGNC -

Paternal Factors and Schizophrenia Risk: De Novo Mutations and Imprinting
Paternal Factors and Schizophrenia Risk: De Novo Mutations and Imprinting by Dolores Malaspina Downloaded from https://academic.oup.com/schizophreniabulletin/article/27/3/379/1835092 by guest on 23 September 2021 Abstract (Impagnatiello et al. 1998; Kao et al. 1998), but there is no consensus that any particular gene plays a meaning- There is a strong genetic component for schizophrenia ful role in the etiology of schizophrenia (Hyman 2000). risk, but it is unclear how the illness is maintained in Some of the obstacles in genetic research in schiz- the population given the significantly reduced fertility ophrenia are those of any complex disorder, and include of those with the disorder. One possibility is that new incomplete penetrance, polygenic interaction (epista- mutations occur in schizophrenia vulnerability genes. sis), diagnostic instability, and variable expressivity. If so, then those with schizophrenia may have older Schizophrenia also does not show a clear Mendelian fathers, because advancing paternal age is the major inheritance pattern, although segregation analyses have source of new mutations in humans. This review variably supported dominant, recessive, additive, sex- describes several neurodevelopmental disorders that linked, and oligogenic inheritance (Book 1953; Slater have been associated with de novo mutations in the 1958; Garrone 1962; Elston and Campbell 1970; Slater paternal germ line and reviews data linking increased and Cowie 1971; Karlsson 1972; Stewart et al. 1980; schizophrenia risk with older fathers. Several genetic Risch 1990a, 1990fc; reviewed by Kendler and Diehl mechanisms that could explain this association are 1993). Furthermore, both nonallelic (Kaufmann et al. proposed, including paternal germ line mutations, 1998) and etiologic heterogeneity (Malaspina et al. -

Bhagwan Moorjani, MD, FAAP, FAAN • Requires Knowledge of Normal CNS Developmental (I.E
1/16/2012 Neuroimaging in Childhood • Neuroimaging issues are distinct from Pediatric Neuroimaging in adults Neurometabolic-degenerative disorder • Sedation/anesthesia and Epilepsy • Motion artifacts Bhagwan Moorjani, MD, FAAP, FAAN • Requires knowledge of normal CNS developmental (i.e. myelin maturation) • Contrast media • Parental anxiety Diagnostic Approach Neuroimaging in Epilepsy • Age of onset • Peak incidence in childhood • Static vs Progressive • Occurs as a co-morbid condition in many – Look for treatable causes pediatric disorders (birth injury, – Do not overlook abuse, Manchausen if all is negative dysmorphism, chromosomal anomalies, • Phenotype presence (syndromic, HC, NCS, developmental delays/regression) systemic involvement) • Predominant symptom (epilepsy, DD, • Many neurologic disorders in children weakness/motor, psychomotor regression, have the same chief complaint cognitive/dementia) 1 1/16/2012 Congenital Malformation • Characterized by their anatomic features • Broad categories: based on embryogenesis – Stage 1: Dorsal Induction: Formation and closure of the neural tube. (Weeks 3-4) – Stage 2: Ventral Induction: Formation of the brain segments and face. (Weeks 5-10) – Stage 3: Migration and Histogenesis: (Months 2-5) – Stage 4: Myelination: (5-15 months; matures by 3 years) Dandy Walker Malformation Dandy walker • Criteria: – high position of tentorium – dysgenesis/agenesis of vermis – cystic dilatation of fourth ventricle • commonly associated features: – hypoplasia of cerebellum – scalloping of inner table of occipital bone • associated abnormalities: – hydrocephalus 75% – dysgenesis of corpus callosum 25% – heterotropia 10% 2 1/16/2012 Etiology of Epilepsy: Developmental and Genetic Classification of Gray Matter Heterotropia Cortical Dysplasia 1. Secondary to abnormal neuronal and • displaced masses of nerve cells • Subependymal glial proliferation/apoptosis (gray matter) heterotropia (most • most common: small nest common) 2. -

An Overview of Lesch-Nyhan Syndrome
An Overview of Lesch-Nyhan Syndrome Abstract Lesch-Nyhan Syndrome is a disorder that strikes the sufferer with debilitating motor and cognitive problems, hyperuricemia, and the urge to do harm to yourself with acts of self-injurious behavior. Research has lead to the discovery of a genetic sequence that results in a defective enzyme, but researchers are still unsure how this leads to the neurological and behavioral problems that are the hallmark of the disorder. Treatments as simple as wearing oven mitts and as complicated as electrical wiring in the brain have been used to help LNS patients, but no cure for the syndrome seems in sight. Lars Sorensen Prof. Stanley Vitello : IDD : 293:522 : Fall 2008 Rutgers University - Graduate School of Education Introduction In the world of developmental and physical disorders none is stranger than Lesch-Nyhan Syndrome. It brings with it a variety of physical ailments but the defining feature of the condition is behavioral. The sufferer seems to be overtaken with an involuntary uncontrollable compulsion to destroy themselves and those around them. In the autumn of 1962, a young mother brought her four year old son to the pediatric emergency room at Johns Hopkins medical center. The boy had previously been diagnosed with cerebral palsy and could not walk or sit up. He was experiencing pain when he urinated. His mother told the resident who was examining the boy that he had “sand in his diaper” (Preston, 2007). The young boy was admitted to the hospital. The resident and an intern began examining the “sand” from the boy's diaper. -

(Hunter Syndrome) Complicated by Autoimmune Hemolytic Anemia
Bone Marrow Transplantation (2000) 25, 1093–1099 2000 Macmillan Publishers Ltd All rights reserved 0268–3369/00 $15.00 www.nature.com/bmt Case report Unrelated umbilical cord blood transplantation in infancy for mucopolysaccharidosis type IIB (Hunter syndrome) complicated by autoimmune hemolytic anemia CA Mullen1,2, JN Thompson3, LA Richard and KW Chan1 Departments of 1Pediatrics and 2Immunology, University of Texas MD Anderson Cancer Center, Houston, Texas; 3Laboratory of Medical Genetics, University of Alabama at Birmingham, Birmingham, Alabama, USA Summary: a median of 21 years in type IIB.8 Allogeneic BMT has been used to treat Hunter disease, but remains controversial This report describes unrelated umbilical cord blood since it often fails to reverse CNS impairment9–11 and car- transplantation for a 10-month-old infant boy with ries with it substantial early mortality and morbidity. Here, mucopolysaccharidosis IIB (Hunter syndrome), an X- we report treatment of an infant with mucopolysacch- linked metabolic storage disorder due to deficiency of arisosis type IIB with transplantation of unrelated umbilical iduronate sulfatase. Two years after transplant ෂ55% cord blood cells and its complication by autoimmune hemo- normal plasma enzyme activity has been restored and lytic anemia. abnormal urinary excretion of glycosaminoglycans has nearly completely resolved. The boy has exhibited nor- mal growth and development after transplant. Nine Case report months after transplant he developed severe auto- immune hemolytic anemia and required 14 months of The patient is the only child of a couple with a maternal corticosteroid treatment to prevent clinically significant family history of Hunter syndrome. The mother’s brother anemia. Bone marrow transplantation for Hunter syn- was diagnosed with Hunter syndrome in 1979 at age 3 years drome and post-transplant hemolytic anemia are when he exhibited the physical stigmata of the disorder. -

Description Treatment
Description Megalencephaly, also called macrencephaly, is a condition in which an infant or child has an abnormally large, heavy, and usually malfunctioning brain. By definition, the brain weight is greater than average for the age and gender of the child. Head enlargement may be evident at birth or the head may become abnormally large in the early years of life. Megalencephaly is thought to be related to a disturbance in the regulation of cell production in the brain. In normal development, neuron proliferation - the process in which nerve cells divide to form new generations of cells - is regulated so that the correct number of cells is produced in the proper place at the appropriate time. In a megalencephalic brain, too many cells are produced either during development or progressively as part of another disorder, such as one of the neurofibromatoses or leukodystrophies. Symptoms of megalencephaly include delayed development, seizures, and corticospinal (brain cortex and spinal cord) dysfunction. Megalencephaly affects males more often than females. Unilateral megalencephaly or hemimegalencephaly is a rare condition that is characterized by the enlargement of one side of the brain. Children with this disorder may have a large, asymmetrical head accompanied by seizures, partial paralysis, and impaired cognitive development. Megalencephaly is different from macrocephaly (also called megacephaly or megalocephaly), which describes a big head, and which doesn’t necessarily indicate abnormality. Large head size is passed down through the generations in some families. Treatment There is no standard treatment for megalencephaly. Treatment will depend upon the disorder with which the megalencephaly is associated and will address individual symptoms and disabilities. -

New Microdeletion and Microduplication Syndromes: a Comprehensive Review
Genetics and Molecular Biology, 37, 1 (suppl), 210-219 (2014) Copyright © 2014, Sociedade Brasileira de Genética. Printed in Brazil www.sbg.org.br Review Article New microdeletion and microduplication syndromes: A comprehensive review Julián Nevado1,2*, Rafaella Mergener3*, María Palomares-Bralo1,2, Karen Regina Souza3, Elena Vallespín1,2, Rocío Mena1,2, Víctor Martínez-Glez1,2, María Ángeles Mori1,2, Fernando Santos1,4, Sixto García-Miñaur1,4, Fé García-Santiago1,5, Elena Mansilla1,5, Luis Fernández1,6, María Luisa de Torres1,5, Mariluce Riegel3,7$ and Pablo Lapunzina1,4,8$ 1Centro de Investigación Biomédica en Red de Enfermedades Raras, Instituto de Salud Carlos III, Madrid, Spain. 2Section of Functional and Structural Genomics, Instituto de Genética Médica y Molecular, Hospital Universitario la Paz, Madrid, Spain. 3Programa de Pós-graduação em Genética e Biologia Molecular, Universidade Federal do Rio Grande do Sul, Porto Alegre,RS, Brazil. 4Section of Clinical Genetics, Instituto de Genética Médica y Molecular, Hospital Universitario la Paz, Madrid, Spain. 5Section of Cytogenetics, Instituto de Genética Médica y Molecular, Hospital Universitario la Paz, Madrid, Spain. 6Section of Preanalytics, Instituto de Genética Médica y Molecular, Hospital Universitario la Paz, Madrid, Spain. 7Serviço de Genética Médica, Hospital de Clínicas de Porto Alegre, Porto Alegre ,RS, Brazil. 8Section of Molecular Endocrinology, Overgrowth Disordes Laboratory, Instituto de Genética Médica y Molecular, Hospital Universitario la Paz, Madrid, Spain. Abstract Several new microdeletion and microduplication syndromes are emerging as disorders that have been proven to cause multisystem pathologies frequently associated with intellectual disability (ID), multiple congenital anomalies (MCA), autistic spectrum disorders (ASD) and other phenotypic findings. In this paper, we review the “new” and emergent microdeletion and microduplication syndromes that have been described and recognized in recent years with the aim of summarizing their main characteristics and chromosomal regions involved. -

Mini-Review on “Molecular Diagnosis of 65 Families With
Mashima R, Okuyama T. J Rare Dis Res Treat. (2016) 2(1): 43-46 Journal of www.rarediseasesjournal.com Rare Diseases Research & Treatment Mini-Review Open Access Mini-review on “Molecular diagnosis of 65 families with mucopoly- saccharidosis type II (Hunter syndrome) characterized by 16 novel mutations in the IDS gene: Genetic, pathological, and structural stud- ies on iduronate-2-sulfatase.” Ryuichi Mashima1* and Torayuki Okuyama1,2 1Department of Clinical Laboratory Medicine, National Center for Child Health and Development, 2-10-1 Okura, Setagaya-ku, Tokyo 157-8535, Japan 2Center for Lysosomal Storage Disorders, National Center for Child Health and Development, 2-10-1 Okura, Setagaya-ku, Tokyo 157-8535, Japan ABSTRACT Article Info Article Notes Mucopolysaccharidosis type II (MPS II; Hunter syndrome; OMIM #309900) Received: November 29, 2016 is an X-linked congenital disorder characterized by an accumulation of Accepted: December 28, 2016 glycosaminoglycans in the body. Accumulating evidence has suggested that the prevalence of the severe type of MPS II is almost 70%. In addition, novel *Correspondence: mutations that are relevant to MPS II pathogenesis are being increasingly Ryuichi Mashima, Department of Clinical Laboratory Medicine, National Center for Child Health and Development, 2-10- discovered, so the databases of genetic data regarding pathogenic mutations 1 Okura, Setagaya-ku, Tokyo 157-8535, Japan, E-mail: have been growing. We have recently reported a collection of 16 novel [email protected] pathogenic mutations of the iduronate-2-sulfatase (IDS) gene in 65 families with MPS II in a Japanese population1. We also proposed that a homology- © 2016 Ryuichi Mashima. -

1Q21.1 Duplication Syndrome and Epilepsy Case Report and Review
CLINICAL/SCIENTIFIC NOTES OPEN ACCESS 1q21.1 Duplication syndrome and epilepsy Case report and review Ioulia Gourari, MD, Romaine Schubert, MD, and Aparna Prasad, PhD Correspondence Dr. Gourari Neurol Genet 2018;4:e219. doi:10.1212/NXG.0000000000000219 [email protected] Copy number variants (CNVs) of 1q21.1 are increasingly being recognized due to the wide- spread use of genetic screening tests for the investigation of developmental disorders and epilepsy. These include microdeletion and microduplication syndromes, associated with a wide variety of pathology including autism spectrum disorders, attention-deficit disorder, learning disabilities, hypotonia, facial dysmorphisms, and schizophrenia. The 1q21.1 region is consid- ered to be genetically unstable because it contains one of the largest areas of identical dupli- cation sequences in the human genome. Epilepsy has been reported in the literature, particularly in microdeletion syndromes, but rarely in association with microduplication syn- dromes. We report a patient with epilepsy and autism spectrum disorder due to a distal 1q21.1 microduplication and review the available literature and genetic information. Case report We present a 10-year-old girl with a low-functioning autism spectrum disorder and focal motor epilepsy. On examination, she has hypertelorism, minimal communicative language skills, and severe macrocephaly (HC = 57 cm, 3.6 SD > 99%). Seizures started at 7 years of age and consisted of head deviation to the left, generalized stiffening, clonic activity of the mouth, and fluttering of the eyelids, lasting for 1–2 minutes. Multiple video EEG recordings showed a right temporal focus with a less active, independent left temporal focus. 3T MRI scan of the brain was normal.