Subcommittee on Natural Assessment of Fundamental Understanding of Isotopes of IUPAC
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Package 'Ciaawconsensus'
Package ‘CIAAWconsensus’ September 19, 2018 Type Package Title Isotope Ratio Meta-Analysis Version 1.3 Author Juris Meija and Antonio Possolo Maintainer Juris Meija <[email protected]> Description Calculation of consensus values for atomic weights, isotope amount ratios, and iso- topic abundances with the associated uncertainties using multivariate meta-regression ap- proach for consensus building. License Unlimited LazyData yes Imports mvtnorm, stringr, numDeriv, stats, Matrix NeedsCompilation no Repository CRAN Date/Publication 2018-09-19 13:30:12 UTC R topics documented: abundances2ratios . .2 at.weight . .3 ciaaw.mass.2003 . .4 ciaaw.mass.2012 . .5 ciaaw.mass.2016 . .6 iridium.data . .6 mmm ............................................7 normalize.ratios . .8 platinum.data . .9 Index 10 1 2 abundances2ratios abundances2ratios Isotope ratios of a chemical element from isotopic abundances Description This function calculates the isotope ratios of a chemical element from the given isotopic abundances and their uncertainties. The uncertainty evaluation is done using the propagation of uncertainty and the missing correlations between the isotopic abundances are reconstructed using Monte Carlo methods. Usage abundances2ratios(x, ux, ref=1, iterations=1e4) Arguments x A vector of isotopic abundances of an element ux Standard uncertainties of x ref Index to specify the desired reference isotope for isotope amount ratios iterations Number of iterations for isotopic abundance correlation mapping Details Situations are often encountered where isotopic abundances are reported but not the isotope ratios. In such cases we reconstruct the isotope ratios that are consistent with the abundances and their uncertainties. Given only the abundances and their uncertainties, for elements with four or more isotopes one cannot unambiguously infer the uncertainties of the ratios due to the unknown correla- tions between isotopic abundances. -

Properties of Carbon the Atomic Element Carbon Has Very Diverse
Properties of Carbon The atomic element carbon has very diverse physical and chemical properties due to the nature of its bonding and atomic arrangement. fig. 1 Allotropes of Carbon Some allotropes of carbon: (a) diamond, (b) graphite, (c) lonsdaleite, (d–f) fullerenes (C60, C540, C70), (g) amorphous carbon, and (h) carbon nanotube. Carbon has several allotropes, or different forms in which it can exist. These allotropes include graphite and diamond, whose properties span a range of extremes. Despite carbon's ability to make 4 bonds and its presence in many compounds, it is highly unreactive under normal conditions. Carbon exists in 2 main isotopes: 12C and 13C. There are many other known isotopes, but they tend to be short-lived and have extremely short half-lives. Allotropes The different forms of a chemical element. Cabon is the chemical element with the symbol C and atomic number 6. As a member of group 14 on the periodic table, it is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds. Carbon has 6 protons and 6 Source URL: https://www.boundless.com/chemistry/nonmetallic-elements/carbon/properties-carbon/ Saylor URL: http://www.saylor.org/courses/chem102#6.1 Attributed to: Boundless www.saylor.org Page 1 of 2 neutrons, and has a standard atomic weight of 12.0107 amu. Its electron configuration is denoted as 1s22s22p2. It is a solid, and sublimes at 3,642 °C. It's oxidation state ranges from 4 to -4, and it has an electronegativity rating of 2.55 on the Pauling scale. Carbon has several allotropes, or different forms in which it exists. -

The Periodic Table of the Elements
The Periodic Table of the Elements The president of the Inorganic Chemistry Division, atomic number was the same as the number of protons Gerd Rosenblatt, recognizing that the periodic table in each element. of the elements found in the “Red Book” A problem for Mendeleev’s table was the position- (Nomenclature of Inorganic Chemistry, published in ing of the rare earth or lanthanoid* elements. These 1985) needed some updating—particularly elements elements had properties and atomic weight values above 103, including element 110 (darmstadtium)— similar to one another but that did not follow the reg- made a formal request to Norman Holden and Tyler ularities of the table. Eventually, they were placed in a Coplen to prepare an updated table. This table can be separate area below the main table. found below, on the IUPAC Web site, and as a tear-off The Danish physicist Niels Henrik David Bohr pro- on the inside back cover of this issue. posed his electronic orbital structure of the atom in 1921, which explained the problem of the rare earth by Norman Holden and Ty Coplen elements. The electrons in the outermost and the penultimate orbits are called valence electrons since generally their actions account for the valence of the he Russian chemist Dmitri Ivanovich Mendeleev element (i.e., electrons capable of taking part in the constructed his original periodic table in 1869 links between atoms). Chemical behavior of an ele- Tusing as its organizing principle his formulation ment depends on its valence electrons, so that when of the periodic law: if the chemical elements are only inner orbit electrons are changing from one ele- arranged in the ascending order of their atomic ment to another, there is not much difference in the weights, then at certain regular intervals (periods) chemical properties between the elements. -

Guidelines for the Use of Atomic Weights 5 10 11 12 DOI: ..., Received ...; Accepted
IUPAC Guidelines for the us e of atomic weights For Peer Review Only Journal: Pure and Applied Chemistry Manuscript ID PAC-REC-16-04-01 Manuscript Type: Recommendation Date Submitted by the Author: 01-Apr-2016 Complete List of Authors: van der Veen, Adriaan; VSL Meija, Juris Possolo, Antonio; National Institute of Standards and Technology Hibbert, David; University of New South Wales, School of Chemistry atomic weights, atomic-weight intervals, molecular weight, standard Keywords: atomic weight, measurement uncertainty, uncertainty propagation Author-Supplied Keywords: P.O. 13757, Research Triangle Park, NC (919) 485-8700 Page 1 of 13 IUPAC Pure Appl. Chem. 2016; aop 1 2 3 4 Sponsoring body: IUPAC Inorganic Chemistry Division Committee: see more details on page XXX. 5 IUPAC Recommendation 6 7 Adriaan M. H. van der Veen*, Juris Meija, Antonio Possolo, and D. Brynn Hibbert 8 9 Guidelines for the use of atomic weights 5 10 11 12 DOI: ..., Received ...; accepted ... 13 14 Abstract: Standard atomicFor weights Peer are widely used Review in science, yet the uncertainties Only associated with these 15 values are not well-understood. This recommendation provides guidance on the use of standard atomic 16 weights and their uncertainties. Furthermore, methods are provided for calculating standard uncertainties 17 of molecular weights of substances. Methods are also outlined to compute material-specific atomic weights 10 18 whose associated uncertainty may be smaller than the uncertainty associated with the standard atomic 19 weights. 20 21 Keywords: atomic weights; atomic-weight intervals; molecular weight; standard atomic weight; uncertainty; 22 uncertainty propagation 23 24 25 1 Introduction 15 26 27 Atomic weights provide a practical link the SI base units kilogram and mole. -

Project Note Weston Solutions, Inc
PROJECT NOTE WESTON SOLUTIONS, INC. To: Canadian Radium & Uranium Corp. Site File Date: June 5, 2014 W.O. No.: 20405.012.013.2222.00 From: Denise Breen, Weston Solutions, Inc. Subject: Determination of Significant Lead Concentrations in Sediment Samples References 1. New York State Department of Environmental Conservation. Technical Guidance for Screening Contaminated Sediments. March 1998. [45 pages] 2. U.S. Environmental Protection Agency (EPA) Office of Emergency Response. Establishing an Observed Release – Quick Reference Fact Sheet. Federal Register, Volume 55, No. 241. September 1995. [7 pages] 3. International Union of Pure and Applied Chemistry, Inorganic Chemistry Division Commission on Atomic Weights and Isotopic Abundances. Atomic Weights of Elements: Review 2000. 2003. [120 pages] WESTON personnel collected six sediment samples (including one environmental duplicate sample) from five locations along the surface water pathway of the Canadian Radium & Uranium Corp. (CRU) site in May 2014. The sediment samples were analyzed for Target Analyte List (TAL) Metals and Stable Lead Isotopes. 1. TAL Lead Interpretation: In order to quantify the significance for Lead, Thallium and Mercury the following was performed: 1. WESTON personnel tabulated all available TAL Metal data from the May 2014 Sediment Sampling event. 2. For each analyte of concern (Lead, Thallium, and Mercury), the highest background concentration was selected and then multiplied by three. This is the criteria to find the significance of site attributable release as per Hazard Ranking System guidelines. 3. One analytical lead result (2222-SD04) of 520 mg/kg (J) was qualified with an unknown bias. In accordance with US EPA document “Using Data to Document an Observed Release and Observed Contamination”, 2222-SD03 lead concentration was adjusted by dividing by the factor value for lead of 1.44 to equal 361 mg/kg. -

Atomic Weights of the Elements 1989
Atomic Weights of the Elements 1989 Cite as: Journal of Physical and Chemical Reference Data 20, 1313 (1991); https://doi.org/10.1063/1.555902 Submitted: 03 June 1991 . Published Online: 15 October 2009 J. R. De Laeter, and K. G. Heumann ARTICLES YOU MAY BE INTERESTED IN The Solubility of Carbon Dioxide in Water at Low Pressure Journal of Physical and Chemical Reference Data 20, 1201 (1991); https:// doi.org/10.1063/1.555900 Chemical Kinetic Data Sheets for High-Temperature Reactions. Part II Journal of Physical and Chemical Reference Data 20, 1211 (1991); https:// doi.org/10.1063/1.555901 Atomic Weights of the Elements 1991 Journal of Physical and Chemical Reference Data 22, 1571 (1993); https:// doi.org/10.1063/1.555933 Journal of Physical and Chemical Reference Data 20, 1313 (1991); https://doi.org/10.1063/1.555902 20, 1313 © 1991 American Institute of Physics for the National Institute of Standards and Technology. a Atomic Weights of the Elements 1989 ) J. R. De Laeter Curtin UlliversityofTechnology, Perth, Western Australia, 6001, Australia K. G. Heumann University ofRegensburg, Regensburg, Germany Received June 3, 1991 The biennial review of atomic weight, A r (E), determinations, and other cognate data has resulted in changes for nickel from 58.69 ± 0.01 to 58.6934 ± 0.0002 and for antimo ny from 121.75 ± 0.03 to 121.757 ± 0.003 due to new calibrated measurements. Because the measurement of the isotopic composition of mercury has also been improved during the last two years, the Commission was able to reduce the uncertainty of the atomic weight of this eJement from 200.59 ± 0.03 to 200.59 ± 0.02. -

Karlsruhe Nuclide Chart – New 10Th Edition 2018
EPJ Nuclear Sci. Technol. 5, 6 (2019) Nuclear © Sciences Z. Sóti et al., published by EDP Sciences, 2019 & Technologies https://doi.org/10.1051/epjn/2019004 Available online at: https://www.epj-n.org REGULAR ARTICLE Karlsruhe Nuclide Chart – New 10th edition 2018 Zsolt Sóti1,*, Joseph Magill2, and Raymond Dreher2 1 European Commission, Joint Research Centre (JRC), PO Box 2340, 76125 Karlsruhe, Germany 2 Nucleonica GmbH, Magdeburger Str. 2, 76139 Karlsruhe, Germany Received: 20 November 2018 / Received in final form: 12 February 2019 / Accepted: 2 April 2019 Abstract. Obtaining nuclear data is an international activity with new and updated data constantly being determined by thousands of scientists at major research centres worldwide. Because of the large amounts of data generated and the formats used to store these data, the field of nuclear data is highly specialised. To make the most important key data more accessible to a wider audience, nuclide charts have been developed. In this article, we present the scientific highlights of the new 10th Edition of the Karlsruhe Nuclide Chart. The main focus of this Chart is to provide structured, accurate information on the half-lives and decay modes, as well as energies of the emitted radiation for over 4000 experimentally observed ground states and isomer nuclides to an interdisciplinary audience. 1 Introduction The new 10th Edition of the Karlsruhe Nuclide Chart contains nuclear data on 4040 experimentally observed The United Nations has designated 2019 as the interna- nuclide ground states and isomers. New data on tional year of the periodic table of chemical elements, 696 nuclides has been included together with information recognizing it as an instrument “central to linking cultural, on 47 new nuclides. -

BNL-79513-2007-CP Standard Atomic Weights Tables 2007 Abridged To
BNL-79513-2007-CP Standard Atomic Weights Tables 2007 Abridged to Four and Five Significant Figures Norman E. Holden Energy Sciences & Technology Department National Nuclear Data Center Brookhaven National Laboratory P.O. Box 5000 Upton, NY 11973-5000 www.bnl.gov Prepared for the 44th IUPAC General Assembly, in Torino, Italy August 2007 Notice: This manuscript has been authored by employees of Brookhaven Science Associates, LLC under Contract No. DE-AC02-98CH10886 with the U.S. Department of Energy. The publisher by accepting the manuscript for publication acknowledges that the United States Government retains a non-exclusive, paid-up, irrevocable, world-wide license to publish or reproduce the published form of this manuscript, or allow others to do so, for United States Government purposes. This preprint is intended for publication in a journal or proceedings. Since changes may be made before publication, it may not be cited or reproduced without the author’s permission. DISCLAIMER This report was prepared as an account of work sponsored by an agency of the United States Government. Neither the United States Government nor any agency thereof, nor any of their employees, nor any of their contractors, subcontractors, or their employees, makes any warranty, express or implied, or assumes any legal liability or responsibility for the accuracy, completeness, or any third party’s use or the results of such use of any information, apparatus, product, or process disclosed, or represents that its use would not infringe privately owned rights. Reference herein to any specific commercial product, process, or service by trade name, trademark, manufacturer, or otherwise, does not necessarily constitute or imply its endorsement, recommendation, or favoring by the United States Government or any agency thereof or its contractors or subcontractors. -

The Atomic Volume, and Are Held There by the (VERY Dense) Nucleus
- formed by subatomic particles: neutrons (neutral), protons (+), electrons (-) - electrons move through the atomic volume, and are held there by the (VERY dense) nucleus The Atom - the number of = 10-15 m subatomic particles determines the properties of atoms (and defines what is He called ªelementsº) = 10-10 m The atoms may be charged or neutral (how?) −27 −25 (how?) mass range: 1.67 × 10 to 4.52 × 10 kg size range: 62 pm (He) to 520 pm (Cs) Is it possible to have atoms of the same element with different mass?(Isotopes!) Is it possible to have atoms of the same element with different number of e-? (ions!) Each element has a unique Z Z = number of p+ What property do radioactive elements share? Can you find other elements usually known as pollutants? Why isotopes? Isotopes are great probes for: - past climate: water can be found as H216O, H217O and H218O but H216O (that is, the light isotope) evaporates preferentially. A signal of high H218O in the ocean (at a given time) indicates that a lot of water had evaporated, but not returned to the ocean! (how could this happen?) - human diet: where does our food come from? different plants have a different isotope content What other uses of isotopes do you know that can have an application in environmental sciences? Jahren and Kraft (2008) *mention UCSB project ªChildren of the cornº Can we get energy from atoms? ... fusion and fission ªThe sun is a mass of incandescent gas Your local (still active) A gigantic nuclear furnace G star Where hydrogen is built into helium At a temperature of millions -

Atomic Weights of the Elements 2011 (IUPAC Technical Report)*
Pure Appl. Chem., Vol. 85, No. 5, pp. 1047–1078, 2013. http://dx.doi.org/10.1351/PAC-REP-13-03-02 © 2013 IUPAC, Publication date (Web): 29 April 2013 Atomic weights of the elements 2011 (IUPAC Technical Report)* Michael E. Wieser1,‡, Norman Holden2, Tyler B. Coplen3, John K. Böhlke3, Michael Berglund4, Willi A. Brand5, Paul De Bièvre6, Manfred Gröning7, Robert D. Loss8, Juris Meija9, Takafumi Hirata10, Thomas Prohaska11, Ronny Schoenberg12, Glenda O’Connor13, Thomas Walczyk14, Shige Yoneda15, and Xiang-Kun Zhu16 1Department of Physics and Astronomy, University of Calgary, Calgary, Canada; 2Brookhaven National Laboratory, Upton, NY, USA; 3U.S. Geological Survey, Reston, VA, USA; 4Institute for Reference Materials and Measurements, Geel, Belgium; 5Max Planck Institute for Biogeochemistry, Jena, Germany; 6Independent Consultant on MiC, Belgium; 7International Atomic Energy Agency, Seibersdorf, Austria; 8Department of Applied Physics, Curtin University of Technology, Perth, Australia; 9National Research Council of Canada, Ottawa, Canada; 10Kyoto University, Kyoto, Japan; 11Department of Chemistry, University of Natural Resources and Applied Life Sciences, Vienna, Austria; 12Institute for Geosciences, University of Tübingen, Tübingen, Germany; 13New Brunswick Laboratory, Argonne, IL, USA; 14Department of Chemistry (Science) and Department of Biochemistry (Medicine), National University of Singapore (NUS), Singapore; 15National Museum of Nature and Science, Tokyo, Japan; 16Chinese Academy of Geological Sciences, Beijing, China Abstract: The biennial review of atomic-weight determinations and other cognate data has resulted in changes for the standard atomic weights of five elements. The atomic weight of bromine has changed from 79.904(1) to the interval [79.901, 79.907], germanium from 72.63(1) to 72.630(8), indium from 114.818(3) to 114.818(1), magnesium from 24.3050(6) to the interval [24.304, 24.307], and mercury from 200.59(2) to 200.592(3). -
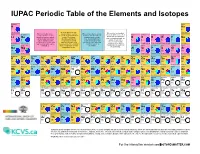
IUPAC Periodic Table of the Elements and Isotopes
IUPAC Periodic Table of the Elements and Isotopes hydrogen helium H He 1 2 1.008 Element has two or more [1.007 84, 1.008 11] Element has no standard 4.002 602(2) Element has two or more isotopes that are used to Element has only one isotope lithium beryllium atomic weight because all of boron carbon nitrogen oxygen fluorine neon isotopes that are used to determine its standard atomic that is used to determine its its isotopes are radioactive determine its atomic weight. weight. The isotopic standard atomic weight. Li Be and, in normal materials, no B C N O F Ne Variations are well known, abundance and atomic Thus, the standard atomic isotope occurs with a 3 4 and the standard atomic weights vary in normal weight is invariant and is 5 6 7 8 9 10 6.94 characteristic isotopic 10.81 12.011 14.007 15.999 weight is given as lower and materials, but upper and given as a single value with [6.938, 6.997] 9.012 1831(5) abundance from which a [10.806, 10.821] [12.0096, 12.0116] [14.006 43, 14.007 28] [15.999 03, 15.999 77] 18.998 403 163(6) 20.1797(6) upper bounds within square lower bounds of the standard an IUPAC evaluated standard atomic weight can sodium magnesium brackets, [ ]. atomic weight have not been uncertainty. aluminium silicon phosphorus sulfur chlorine argon be determined. Na Mg assigned by IUPAC. Al Si P S Cl Ar 11 12 13 14 15 16 17 18 24.305 28.085 32.06 35.45 39.95 22.989 769 28(2) [24.304, 24.307] 26.981 5385(7) [28.084, 28.086] 30.973 761 998(5) [32.059, 32.076] [35.446, 35.457] [39.792, 39.963] potassium calcium scandium titanium -

Atomic Weights
Atomic Weights Just 20 years ago, while completing his chairmanship of Manchester Literacy and Philosophical Society, and the IUPAC Commission on Atomic Weights, Norman than published in 1805.) Since hydrogen had the Holden prepared and published in Chemistry smallest atomic weight value, Dalton chose that ele- International (1984, issue No.1) an early historical review ment as his reference scale unit, hydrogen = 1, and he of the International Commission on Atomic Weights. calculated atomic weights by comparing weights of Since then, his interest of the topic has not faded, and other atoms with that of hydrogen. Values given in au contraire, he has now reviewed, extended, and the table indicate that Dalton grasped the ideas of updated the historical review to a length far beyond the constant composition in compounds and of multiple space available here. The excerpts below are extracted proportions. However, he did not account for the from the historical review, which is available online at valence of each element in the compound. Dalton’s <www.iupac.org/publications/ci/2004/2601/ later tables, published in 1808 and 1810, show a 1_holden.html>. The full text is equivalent to about 20 marked improvement in accuracy but the values are pages and includes more than 100 references. still difficult to recognize because of these errors in valence (i.e., some equivalent weights [atomic Atomic Weights and the weight/valence] are quoted rather than atomic International Committee: weights). By the end of the nineteenth century, atomic A Brief Historical Review weight had taken on the concept of a constant of nature like the speed of light, but the lack of agree- ment on accepted values created difficulties in trade.