Kidney Structure and Function
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Te2, Part Iii
TERMINOLOGIA EMBRYOLOGICA Second Edition International Embryological Terminology FIPAT The Federative International Programme for Anatomical Terminology A programme of the International Federation of Associations of Anatomists (IFAA) TE2, PART III Contents Caput V: Organogenesis Chapter 5: Organogenesis (continued) Systema respiratorium Respiratory system Systema urinarium Urinary system Systemata genitalia Genital systems Coeloma Coelom Glandulae endocrinae Endocrine glands Systema cardiovasculare Cardiovascular system Systema lymphoideum Lymphoid system Bibliographic Reference Citation: FIPAT. Terminologia Embryologica. 2nd ed. FIPAT.library.dal.ca. Federative International Programme for Anatomical Terminology, February 2017 Published pending approval by the General Assembly at the next Congress of IFAA (2019) Creative Commons License: The publication of Terminologia Embryologica is under a Creative Commons Attribution-NoDerivatives 4.0 International (CC BY-ND 4.0) license The individual terms in this terminology are within the public domain. Statements about terms being part of this international standard terminology should use the above bibliographic reference to cite this terminology. The unaltered PDF files of this terminology may be freely copied and distributed by users. IFAA member societies are authorized to publish translations of this terminology. Authors of other works that might be considered derivative should write to the Chair of FIPAT for permission to publish a derivative work. Caput V: ORGANOGENESIS Chapter 5: ORGANOGENESIS -

Excretory Products and Their Elimination
290 BIOLOGY CHAPTER 19 EXCRETORY PRODUCTS AND THEIR ELIMINATION 19.1 Human Animals accumulate ammonia, urea, uric acid, carbon dioxide, water Excretory and ions like Na+, K+, Cl–, phosphate, sulphate, etc., either by metabolic System activities or by other means like excess ingestion. These substances have to be removed totally or partially. In this chapter, you will learn the 19.2 Urine Formation mechanisms of elimination of these substances with special emphasis on 19.3 Function of the common nitrogenous wastes. Ammonia, urea and uric acid are the major Tubules forms of nitrogenous wastes excreted by the animals. Ammonia is the most toxic form and requires large amount of water for its elimination, 19.4 Mechanism of whereas uric acid, being the least toxic, can be removed with a minimum Concentration of loss of water. the Filtrate The process of excreting ammonia is Ammonotelism. Many bony fishes, 19.5 Regulation of aquatic amphibians and aquatic insects are ammonotelic in nature. Kidney Function Ammonia, as it is readily soluble, is generally excreted by diffusion across 19.6 Micturition body surfaces or through gill surfaces (in fish) as ammonium ions. Kidneys do not play any significant role in its removal. Terrestrial adaptation 19.7 Role of other necessitated the production of lesser toxic nitrogenous wastes like urea Organs in and uric acid for conservation of water. Mammals, many terrestrial Excretion amphibians and marine fishes mainly excrete urea and are called ureotelic 19.8 Disorders of the animals. Ammonia produced by metabolism is converted into urea in the Excretory liver of these animals and released into the blood which is filtered and System excreted out by the kidneys. -

Kidney Function • Filtration • Reabsorption • Secretion • Excretion • Micturition
About This Chapter • Functions of the kidneys • Anatomy of the urinary system • Overview of kidney function • Filtration • Reabsorption • Secretion • Excretion • Micturition © 2016 Pearson Education, Inc. Functions of the Kidneys • Regulation of extracellular fluid volume and blood pressure • Regulation of osmolarity • Maintenance of ion balance • Homeostatic regulation of pH • Excretion of wastes • Production of hormones © 2016 Pearson Education, Inc. Anatomy of the Urinary System • Kidneys, ureters, bladder, and urethra • Kidneys – Bean-shaped organ – Cortex and medulla © 2016 Pearson Education, Inc. Anatomy of the Urinary System • Functional unit is the nephron – Glomerulus in the Bowman’s capsule – Proximal tubule – The loop of Henle • Descending limb and ascending limb twisted between arterioles forming the juxtaglomerular apparatus – Distal tubule – Collecting ducts © 2016 Pearson Education, Inc. Figure 19.1b Anatomy summary The kidneys are located retroperitoneally at the level of the lower ribs. Inferior Diaphragm vena cava Aorta Left adrenal gland Left kidney Right kidney Renal artery Renal vein Ureter Peritoneum Urinary Rectum (cut) bladder (cut) © 2016 Pearson Education, Inc. Figure 19.1c Anatomy summary © 2016 Pearson Education, Inc. Figure 19.1d Anatomy summary © 2016 Pearson Education, Inc. Figure 19.1f-h Anatomy summary Some nephrons dip deep into the medulla. One nephron has two arterioles and two sets of capillaries that form a portal system. Efferent arteriole Arterioles Peritubular Juxtaglomerular capillaries The cortex apparatus contains all Bowman’s Nephrons Afferent capsules, arteriole Glomerulus proximal Juxtamedullary nephron and distal (capillaries) with vasa recta tubules. Peritubular capillaries Glomerulus The medulla contains loops of Henle and Vasa recta collecting ducts. Collecting duct Loop of Henle © 2016 Pearson Education, Inc. -

Nomina Histologica Veterinaria, First Edition
NOMINA HISTOLOGICA VETERINARIA Submitted by the International Committee on Veterinary Histological Nomenclature (ICVHN) to the World Association of Veterinary Anatomists Published on the website of the World Association of Veterinary Anatomists www.wava-amav.org 2017 CONTENTS Introduction i Principles of term construction in N.H.V. iii Cytologia – Cytology 1 Textus epithelialis – Epithelial tissue 10 Textus connectivus – Connective tissue 13 Sanguis et Lympha – Blood and Lymph 17 Textus muscularis – Muscle tissue 19 Textus nervosus – Nerve tissue 20 Splanchnologia – Viscera 23 Systema digestorium – Digestive system 24 Systema respiratorium – Respiratory system 32 Systema urinarium – Urinary system 35 Organa genitalia masculina – Male genital system 38 Organa genitalia feminina – Female genital system 42 Systema endocrinum – Endocrine system 45 Systema cardiovasculare et lymphaticum [Angiologia] – Cardiovascular and lymphatic system 47 Systema nervosum – Nervous system 52 Receptores sensorii et Organa sensuum – Sensory receptors and Sense organs 58 Integumentum – Integument 64 INTRODUCTION The preparations leading to the publication of the present first edition of the Nomina Histologica Veterinaria has a long history spanning more than 50 years. Under the auspices of the World Association of Veterinary Anatomists (W.A.V.A.), the International Committee on Veterinary Anatomical Nomenclature (I.C.V.A.N.) appointed in Giessen, 1965, a Subcommittee on Histology and Embryology which started a working relation with the Subcommittee on Histology of the former International Anatomical Nomenclature Committee. In Mexico City, 1971, this Subcommittee presented a document entitled Nomina Histologica Veterinaria: A Working Draft as a basis for the continued work of the newly-appointed Subcommittee on Histological Nomenclature. This resulted in the editing of the Nomina Histologica Veterinaria: A Working Draft II (Toulouse, 1974), followed by preparations for publication of a Nomina Histologica Veterinaria. -

The Urinary System Dr
The urinary System Dr. Ali Ebneshahidi Functions of the Urinary System • Excretion – removal of waste material from the blood plasma and the disposal of this waste in the urine. • Elimination – removal of waste from other organ systems - from digestive system – undigested food, water, salt, ions, and drugs. + - from respiratory system – CO2,H , water, toxins. - from skin – water, NaCl, nitrogenous wastes (urea , uric acid, ammonia, creatinine). • Water balance -- kidney tubules regulate water reabsorption and urine concentration. • regulation of PH, volume, and composition of body fluids. • production of Erythropoietin for hematopoieseis, and renin for blood pressure regulation. Anatomy of the Urinary System Gross anatomy: • kidneys – a pair of bean – shaped organs located retroperitoneally, responsible for blood filtering and urine formation. • Renal capsule – a layer of fibrous connective tissue covering the kidneys. • Renal cortex – outer region of the kidneys where most nephrons is located. • Renal medulla – inner region of the kidneys where some nephrons is located, also where urine is collected to be excreted outward. • Renal calyx – duct – like sections of renal medulla for collecting urine from nephrons and direct urine into renal pelvis. • Renal pyramid – connective tissues in the renal medulla binding various structures together. • Renal pelvis – central urine collecting area of renal medulla. • Hilum (or hilus) – concave notch of kidneys where renal artery, renal vein, urethra, nerves, and lymphatic vessels converge. • Ureter – a tubule that transport urine (mainly by peristalsis) from the kidney to the urinary bladder. • Urinary bladder – a spherical storage organ that contains up to 400 ml of urine. • Urethra – a tubule that excretes urine out of the urinary bladder to the outside, through the urethral orifice. -

Single-Cell Transcriptome Profiling of the Kidney Glomerulus Identifies Key Cell Types and Reactions to Injury
BASIC RESEARCH www.jasn.org Single-Cell Transcriptome Profiling of the Kidney Glomerulus Identifies Key Cell Types and Reactions to Injury Jun-Jae Chung ,1 Leonard Goldstein ,2 Ying-Jiun J. Chen,2 Jiyeon Lee ,1 Joshua D. Webster,3 Merone Roose-Girma,2 Sharad C. Paudyal,4 Zora Modrusan,2 Anwesha Dey,5 and Andrey S. Shaw1 Due to the number of contributing authors, the affiliations are listed at the end of this article. ABSTRACT Background The glomerulus is a specialized capillary bed that is involved in urine production and BP control. Glomerular injury is a major cause of CKD, which is epidemic and without therapeutic options. Single-cell transcriptomics has radically improved our ability to characterize complex organs, such as the kidney. Cells of the glomerulus, however, have been largely underrepresented in previous single-cell kidney studies due to their paucity and intractability. Methods Single-cell RNA sequencing comprehensively characterized the types of cells in the glomerulus from healthy mice and from four different disease models (nephrotoxic serum nephritis, diabetes, doxo- rubicin toxicity, and CD2AP deficiency). Results Allcelltypesintheglomeruluswereidentified using unsupervised clustering analysis. Novel marker genes and gene signatures of mesangial cells, vascular smooth muscle cells of the afferent and efferent arteri- oles, parietal epithelial cells, and three types of endothelial cells were identified. Analysis of the disease models revealed cell type–specific and injury type–specific responses in the glomerulus, including acute activation of the Hippo pathway in podocytes after nephrotoxic immune injury. Conditional deletion of YAP or TAZ resulted in more severe and prolonged proteinuria in response to injury, as well as worse glomerulosclerosis. -
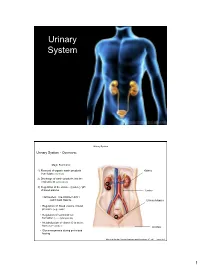
Urinary System
Urinary System Urinary System Urinary System - Overview: Major Functions: 1) Removal of organic waste products Kidney from fluids (excretion) 2) Discharge of waste products into the environment (elimination) 1 3) Regulation of the volume / [solute] / pH 3 of blood plasma Ureter HOWEVER, THE KIDNEY AIN’T JUST FOR PEE’IN… Urinary bladder • Regulation of blood volume / blood pressure (e.g., renin) • Regulation of red blood cell formation (i.e., erythropoietin) 2 • Metabolization of vitamin D to active form (Ca++ uptake) Urethra • Gluconeogenesis during prolonged fasting Marieb & Hoehn (Human Anatomy and Physiology, 8th ed.) – Figure 25.1 1 Urinary System Renal ptosis: Kidneys drop to lower position due Functional Anatomy - Kidney: to loss of perirenal fat Located in the superior lumbar “Bar of soap” region 12 cm x 6 cm x 3 cm 150 g / kidney Layers of Supportive Tissue: Renal fascia: Peritoneal cavity Outer layer of dense fibrous connective tissue; anchors kidney in place Perirenal fat capsule: Fatty mass surrounding kidney; cushions kidney against blows Fibrous capsule: Transparent capsule on kidney; prevents infection of kidney from local tissues Kidneys are located retroperitoneal Marieb & Hoehn (Human Anatomy and Physiology, 8th ed.) – Figure 25.2 Urinary System Functional Anatomy - Kidney: Pyelonephritis: Inflammation of the kidney Pyramids appear striped due to parallel arrangement of capillaries / collecting tubes Renal cortex Renal medulla Renal pyramids Renal papilla Renal columns Renal hilum Renal pelvis • Entrance for blood vessels -

Urinary System A&P
URINARY SYSTEM A&P HS1 DHO8, CH. 7, PGS 217-220 OBJECTIVES EXPLAIN THE STRUCTURES AND FUNCTIONS OF THE URINARY SYSTEM AS THEY RELATE TO THE FORMATION, COMPOSITION, AND ELIMINATION OF URINE. A. IDENTIFY THE STRUCTURES COMPRISING THE URINARY SYSTEM. B. DESCRIBE THE ROLES OF EACH OF THE URINARY STRUCTURES AS IT RELATES TO THE PRODUCTION AND ELIMINATION OF URINE. URINARY SYSTEM • AKA EXCRETORY SYSTEM • REMOVES WASTES & EXCESS WATER • MAINTAIN ACID-BASE BALANCE • HELPS MAINTAIN BODY’S HOMEOSTASIS URINARY SYSTEM PARTS OF THE URINARY SYSTEM: ➢2 KIDNEYS ➢2 URETERS ➢1 BLADDER ➢1 URETHRA KIDNEYS • BEAN-SHAPED ORGANS • FOUND ON EITHER SIDE OF VERTEBRAL COLUMN • LOCATED IN RETROPERITONEAL SPACE • RETROPERITONEAL SPACE=AREA BEHIND UPPER PART OF ABD CAVITY; SEPARATED FROM ABD CAVITY BY PERITONEAL MEMBRANE KIDNEYS • PROTECTED BY RIBS & FAT CUSHION • HELD IN PLACE BY CONNECTIVE TISSUE • EACH KIDNEY IS ENCLOSED IN MASS OF FATTY TISSUE=ADIPOSE CAPSULE • EACH KIDNEY IS COVERED BY A TOUGH, FIBROUS TISSUE=RENAL FASCIA OR FIBROUS CAPSULE APPLY YOUR KNOWLEDGE CAN YOU THINK OF AN EXAMPLE OF THE URINARY SYSTEM’S ABILITY TO MAINTAIN HOMEOSTASIS? ➢A GOOD EXAMPLE IS WHEN A PERSON DRINKS A LARGE AMOUNT OF WATER AND URINARY OUTPUT INCREASES KIDNEYS DIVIDED INTO 2 MAIN SECTIONS: CORTEX & MEDULLA ➢CORTEX= • OUTER SECTION • CONTAINS MOST OF THE NEPHRONS (NEPHRONS AID IN PRODUCTION OF URINE) KIDNEYS ➢MEDULLA= • INNER SECTION • CONTAINS MOST OF THE COLLECTING TUBULES (COLLECTING TUBULES CARRY URINE FROM NEPHRONS THROUGH THE KIDNEY) KIDNEYS • EACH KIDNEY HAS A HILUM • HILUM=NOTCHED OR INDENTED AREA • THE URETER, NERVES, BLOOD VESSELS, & LYMPH VESSELS ENTER & LEAVE THE KIDNEY THROUGH THE HILUM TEST YOUR KNOWLEDGE SO, LET’S THINK THIS THROUGH….YOU HAVE TO PRODUCE THE URINE FIRST AND THEN SEND THE URINE OUT OF THE KIDNEY. -

A Single-Cell Transcriptome Atlas of the Mouse Glomerulus
RAPID COMMUNICATION www.jasn.org A Single-Cell Transcriptome Atlas of the Mouse Glomerulus Nikos Karaiskos,1 Mahdieh Rahmatollahi,2 Anastasiya Boltengagen,1 Haiyue Liu,1 Martin Hoehne ,2 Markus Rinschen,2,3 Bernhard Schermer,2,4,5 Thomas Benzing,2,4,5 Nikolaus Rajewsky,1 Christine Kocks ,1 Martin Kann,2 and Roman-Ulrich Müller 2,4,5 Due to the number of contributing authors, the affiliations are listed at the end of this article. ABSTRACT Background Three different cell types constitute the glomerular filter: mesangial depending on cell location relative to the cells, endothelial cells, and podocytes. However, to what extent cellular heteroge- glomerular vascular pole.3 Because BP ad- neity exists within healthy glomerular cell populations remains unknown. aptation and mechanoadaptation of glo- merular cells are key determinants of kidney Methods We used nanodroplet-based highly parallel transcriptional profiling to function and dysregulated in kidney disease, characterize the cellular content of purified wild-type mouse glomeruli. we tested whether glomerular cell type sub- Results Unsupervised clustering of nearly 13,000 single-cell transcriptomes identi- sets can be identified by single-cell RNA fied the three known glomerular cell types. We provide a comprehensive online sequencing in wild-type glomeruli. This atlas of gene expression in glomerular cells that can be queried and visualized using technique allows for high-throughput tran- an interactive and freely available database. Novel marker genes for all glomerular scriptome profiling of individual cells and is cell types were identified and supported by immunohistochemistry images particularly suitable for identifying novel obtained from the Human Protein Atlas. -

The Distal Convoluted Tubule and Collecting Duct
Chapter 23 *Lecture PowerPoint The Urinary System *See separate FlexArt PowerPoint slides for all figures and tables preinserted into PowerPoint without notes. Copyright © The McGraw-Hill Companies, Inc. Permission required for reproduction or display. Introduction • Urinary system rids the body of waste products. • The urinary system is closely associated with the reproductive system – Shared embryonic development and adult anatomical relationship – Collectively called the urogenital (UG) system 23-2 Functions of the Urinary System • Expected Learning Outcomes – Name and locate the organs of the urinary system. – List several functions of the kidneys in addition to urine formation. – Name the major nitrogenous wastes and identify their sources. – Define excretion and identify the systems that excrete wastes. 23-3 Functions of the Urinary System Copyright © The McGraw-Hill Companies, Inc. Permission required for reproduction or display. Diaphragm 11th and 12th ribs Adrenal gland Renal artery Renal vein Kidney Vertebra L2 Aorta Inferior vena cava Ureter Urinary bladder Urethra Figure 23.1a,b (a) Anterior view (b) Posterior view • Urinary system consists of six organs: two kidneys, two ureters, urinary bladder, and urethra 23-4 Functions of the Kidneys • Filters blood plasma, separates waste from useful chemicals, returns useful substances to blood, eliminates wastes • Regulate blood volume and pressure by eliminating or conserving water • Regulate the osmolarity of the body fluids by controlling the relative amounts of water and solutes -
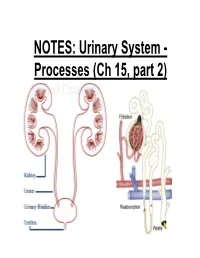
NOTES: Urinary System
NOTES: Urinary System - Processes (Ch 15, part 2) *Recall: the primary function of the urinary system is to filter the blood of ions and nitrogenous wastes; when combined with water, these wastes make up URINE. • Kidneys receive about 20-25% of total cardiac output – ~1200mL of blood goes through the kidneys per minute Blood Supply to Kidneys • Descending aorta Renal artery interlobar arcuate cortical afferent arterioles • The afferent arterioles deliver blood to nephrons NEPHRONS • NEPHRONS: the functional units of the kidneys -each kidney contains about a million nephrons! Parts of a NEPHRON: • GLOMERULUS: tangled cluster of blood capillaries • GLOMERULAR CAPSULE (a.k.a. Bowman’s capsule): thin-walled structure surrounding glomerulus Parts of a NEPHRON: • PROXIMAL CONVOLUTED TUBULE • LOOP OF HENLE -descending limb -ascending limb • DISTAL CONVOLUTED TUBULE Parts of a NEPHRON: • COLLECTING DUCT (where distal tubules from several nephrons converge and drain into; from here, urine empties into the RENAL PELVIS) Blood Supply of a Nephron: -blood is brought to a nephron from an afferent arteriole; -from here, it is passed to an efferent arteriole; -this gives rise to a system of peritubular capillaries that surround the renal tubules URINE FORMATION *nephrons remove wastes from blood and regulate water and electrolyte concentrations. URINE IS THE END PRODUCT! Three Organic Wastes Products of Urine 1) Urea: most abundant, from breakdown of amino acids 2) Creatinine: generated in skeletal muscle when creatine phosphate is broken down (creatine -

Urine-Derived Epithelial Cells As Models for Genetic Kidney Diseases
cells Review Urine-Derived Epithelial Cells as Models for Genetic Kidney Diseases Tjessa Bondue 1 , Fanny O. Arcolino 1 , Koenraad R. P. Veys 1,2, Oyindamola C. Adebayo 1,3, Elena Levtchenko 1,2, Lambertus P. van den Heuvel 1,4 and Mohamed A. Elmonem 5,* 1 Department of Development and Regeneration, KU Leuven, 3000 Leuven, Belgium; [email protected] (T.B.); [email protected] (F.O.A.); [email protected] (K.R.P.V.); [email protected] (O.C.A.); [email protected] (E.L.); [email protected] (L.P.v.d.H.) 2 Department of Pediatrics, Division of Pediatric Nephrology, University Hospitals Leuven, 3000 Leuven, Belgium 3 Centre for Molecular and Vascular Biology, Department of Cardiovascular Sciences, KU Leuven, 3000 Leuven, Belgium 4 Department of Pediatric Nephrology, Radboud University Medical Center, 6500 Nijmegen, The Netherlands 5 Department of Clinical and Chemical Pathology, Faculty of Medicine, Cairo University, Cairo 11628, Egypt * Correspondence: [email protected] Abstract: Epithelial cells exfoliated in human urine can include cells anywhere from the urinary tract and kidneys; however, podocytes and proximal tubular epithelial cells (PTECs) are by far the most relevant cell types for the study of genetic kidney diseases. When maintained in vitro, they have been proven extremely valuable for discovering disease mechanisms and for the development of new therapies. Furthermore, cultured patient cells can individually represent their human sources and their specific variants for personalized medicine studies, which are recently gaining much Citation: Bondue, T.; Arcolino, F.O.; interest. In this review, we summarize the methodology for establishing human podocyte and PTEC Veys, K.R.P.; Adebayo, O.C.; cell lines from urine and highlight their importance as kidney disease cell models.