The Development of the Palate – a Brief Review
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Te2, Part Iii
TERMINOLOGIA EMBRYOLOGICA Second Edition International Embryological Terminology FIPAT The Federative International Programme for Anatomical Terminology A programme of the International Federation of Associations of Anatomists (IFAA) TE2, PART III Contents Caput V: Organogenesis Chapter 5: Organogenesis (continued) Systema respiratorium Respiratory system Systema urinarium Urinary system Systemata genitalia Genital systems Coeloma Coelom Glandulae endocrinae Endocrine glands Systema cardiovasculare Cardiovascular system Systema lymphoideum Lymphoid system Bibliographic Reference Citation: FIPAT. Terminologia Embryologica. 2nd ed. FIPAT.library.dal.ca. Federative International Programme for Anatomical Terminology, February 2017 Published pending approval by the General Assembly at the next Congress of IFAA (2019) Creative Commons License: The publication of Terminologia Embryologica is under a Creative Commons Attribution-NoDerivatives 4.0 International (CC BY-ND 4.0) license The individual terms in this terminology are within the public domain. Statements about terms being part of this international standard terminology should use the above bibliographic reference to cite this terminology. The unaltered PDF files of this terminology may be freely copied and distributed by users. IFAA member societies are authorized to publish translations of this terminology. Authors of other works that might be considered derivative should write to the Chair of FIPAT for permission to publish a derivative work. Caput V: ORGANOGENESIS Chapter 5: ORGANOGENESIS -
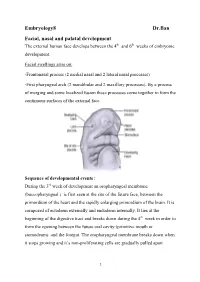
Embryology8 Dr.Ban Facial, Nasal and Palatal Development the External Human Face Develops Between the 4Th and 6Th Weeks of Embryonic Development
Embryology8 Dr.Ban Facial, nasal and palatal development The external human face develops between the 4th and 6th weeks of embryonic development. Facial swellings arise on: -Frontonasal process (2 medial nasal and 2 lateral nasal processes) -First pharyngeal arch (2 mandibular and 2 maxillary processes). By a process of merging and some localized fusion these processes come together to form the continuous surfaces of the external face. Sequence of developmental events : During the 3rd week of development an oropharyngeal membrane (buccopharyngeal ) is first seen at the site of the future face, between the primordium of the heart and the rapidly enlarging primordium of the brain. It is composed of ectoderm externally and endoderm internally. It lies at the beginning of the digestive tract and breaks down during the 4th week in order to form the opening between the future oral cavity (primitive mouth or stomodeum) and the foregut. The oropharyngeal membrane breaks down when it stops growing and it’s non-proliferating cells are gradually pulled apart 1 because they cannot fill the expanding area.The tissues around it expand very rapidly. The face develops from five primordia that appear in the fourth week: the frontonasal prominence, the two maxillary swellings, and the two mandibular swellings. The external face forms from two sources that surround the oropharyngeal membrane 1-Tissues of the frontonasal process that cover the forebrain, predominantly of neural crest origin. 2-The tissues of the first (or mandibular) pharyngeal arch, of mixed mesoderm and neural crest origin Face initially formed by 5 mesenchymal swellings ( prominences): Two mandibular prominences Two maxillary prominences Frontonasal prominence (midline structure is a single structure that is ventral to the forebrain. -

Vocabulario De Morfoloxía, Anatomía E Citoloxía Veterinaria
Vocabulario de Morfoloxía, anatomía e citoloxía veterinaria (galego-español-inglés) Servizo de Normalización Lingüística Universidade de Santiago de Compostela COLECCIÓN VOCABULARIOS TEMÁTICOS N.º 4 SERVIZO DE NORMALIZACIÓN LINGÜÍSTICA Vocabulario de Morfoloxía, anatomía e citoloxía veterinaria (galego-español-inglés) 2008 UNIVERSIDADE DE SANTIAGO DE COMPOSTELA VOCABULARIO de morfoloxía, anatomía e citoloxía veterinaria : (galego-español- inglés) / coordinador Xusto A. Rodríguez Río, Servizo de Normalización Lingüística ; autores Matilde Lombardero Fernández ... [et al.]. – Santiago de Compostela : Universidade de Santiago de Compostela, Servizo de Publicacións e Intercambio Científico, 2008. – 369 p. ; 21 cm. – (Vocabularios temáticos ; 4). - D.L. C 2458-2008. – ISBN 978-84-9887-018-3 1.Medicina �������������������������������������������������������������������������veterinaria-Diccionarios�������������������������������������������������. 2.Galego (Lingua)-Glosarios, vocabularios, etc. políglotas. I.Lombardero Fernández, Matilde. II.Rodríguez Rio, Xusto A. coord. III. Universidade de Santiago de Compostela. Servizo de Normalización Lingüística, coord. IV.Universidade de Santiago de Compostela. Servizo de Publicacións e Intercambio Científico, ed. V.Serie. 591.4(038)=699=60=20 Coordinador Xusto A. Rodríguez Río (Área de Terminoloxía. Servizo de Normalización Lingüística. Universidade de Santiago de Compostela) Autoras/res Matilde Lombardero Fernández (doutora en Veterinaria e profesora do Departamento de Anatomía e Produción Animal. -

Development and Malformations of Face and Palate
Development and malformations of face and palate Compiled by András Csillag and Andrea D. Székely GERMINAL LAYER DERIVATIVES ECTODERM contributing to the formation of the face appears by the 4th week. The oropharyngeal membrane (interface between ECTODERM and ENDODERM) is located in front of the later palatine tonsils. Ectodermal structures limiting the stomodeum participate in the formation of the face, as well as of the nasal and oral cavities. MESENCHYME that fills the pharyngeal arches derives from the neural crest ECTOMESENCHYME DEVELOPMENT OF THE FACE WEEK 6 The face is formed by 5 processes approximately 24 days Frontonasal prominence (1) Maxillary prominence (2) 1st arch Mandibular prominence (2) nasal (olfactory) pits form surrounded by the medial and lateral nasal processes nasolacrimal groove separates the lateral nasal process from the maxillary process maxillary processes fuse with the medial nasal processes lateral nasal processes fuse with the maxillary processes, thus obliterating the nasolacrimal groove. DEVELOPMENT OF THE FACE 5-week embryo 6-week embryo 7-week embryo. 10-week embryo The nasal prominences are gradually Maxillary prominences have fused separated from the maxillary with the medial nasal prominences. prominence by deep furrows. CEPHALIC PRIMORDIA - PLACODES Olfactory placode Pharyngeal Stomodeum” arches Nasal placodes - thickenings of the surface ectoderm (later differentiate into the olfactory epithelium) DEVELOPMENT OF THE NASAL CAVITIES AND THE HARD PALATE By week 5 the placodes form the nasal pits. They further invaginate and the pits approach the primitive oral cavity. A thin oronasal membrane separates the two cavities. By its rupture the primitive choanae will form DEVELOPMENT OF THE NASAL CAVITIES AND THE HARD PALATE By week 8, a partition forms between the primitive nasal chambers and the oral cavity The primary palate - the anterior aspect derives from the intermaxillary segment (or median palatine process, formed by the medial nasal processes). -

Syndromes of the First and Second Branchial Arches, Part 1: Embryology and Characteristic REVIEW ARTICLE Defects
Syndromes of the First and Second Branchial Arches, Part 1: Embryology and Characteristic REVIEW ARTICLE Defects J.M. Johnson SUMMARY: A variety of congenital syndromes affecting the face occur due to defects involving the G. Moonis first and second BAs. Radiographic evaluation of craniofacial deformities is necessary to define aberrant anatomy, plan surgical procedures, and evaluate the effects of craniofacial growth and G.E. Green surgical reconstructions. High-resolution CT has proved vital in determining the nature and extent of R. Carmody these syndromes. The radiologic evaluation of syndromes of the first and second BAs should begin H.N. Burbank first by studying a series of isolated defects: CL with or without CP, micrognathia, and EAC atresia, which compose the major features of these syndromes and allow more specific diagnosis. After discussion of these defects and the associated embryology, we proceed to discuss the VCFS, PRS, ACS, TCS, Stickler syndrome, and HFM. ABBREVIATIONS: ACS ϭ auriculocondylar syndrome; BA ϭ branchial arch; CL ϭ cleft lip; CL/P ϭ cleft lip/palate; CP ϭ cleft palate; EAC ϭ external auditory canal; HFM ϭ hemifacial microsomia; MDCT ϭ multidetector CT; PRS ϭ Pierre Robin sequence; TCS ϭ Treacher Collins syndrome; VCFS ϭ velocardiofacial syndrome adiographic evaluation of craniofacial deformities is nec- major features of the syndromes of the first and second BAs. Ressary to define aberrant anatomy, plan surgical proce- Part 2 of this review discusses the syndromes and their radio- dures, and evaluate the effects of craniofacial growth and sur- graphic features: PRS, HFM, ACS, TCS, Stickler syndrome, gical reconstructions.1 The recent rapid proliferation of and VCFS. -

Illustrated Review of the Embryology and Development of the Facial
REVIEW ARTICLE Illustrated Review of the Embryology and Development of the Facial Region, Part 2: Late Development of the Fetal Face and Changes in the Face from the Newborn to Adulthood P.M. Som and T.P. Naidich ABSTRACT SUMMARY: The later embryogenesis of the fetal face and the alteration in the facial structure from birth to adulthood have been reviewed. Part 3 of the review will address the molecular mechanisms that are responsible for the changes described in parts 1 and 2. art 1 of this 3-part review primarily dealt with the early em- first make contact, each is completely covered by a homoge- Pbryologic development of the face and nasal cavity. Part 2 will neous epithelium. A special epithelium arises at the edge of discuss the later embryonic and fetal development of the face, and each palatal shelf, facilitating the eventual fusion of these changes in facial appearance from neonate to adulthood will be shelves. The epithelium on the nasal cavity surface of the palate reviewed. will differentiate into columnar ciliated epithelium. The epi- thelium on the oral cavity side of the palate will differentiate Formation of the Palate into stratified squamous epithelium. Between the sixth and 12th weeks, the palate is formed from 3 The 2 palatal shelves also fuse with the triangular primary pal- primordia: a midline median palatine process and paired lateral ate anteromedially to form a y-shaped fusion line. The point of palatine processes (Fig 1). In the beginning of the sixth week, fusion of the secondary palatal shelves with the primary palate is merging of the paired medial nasal processes forms the intermax- marked in the adult by the incisive foramen. -

EMBRYOLOGY7 Dr.Ban A.Ghani Developmdent of External
EMBRYOLOGY7 Dr.Ban A.Ghani EMBRYOLOGY7 Dr.Ban A.Ghani Developmdent of external /Sequence of developmental During the third week of development an oropharyngeal membrane buccopharyngeal , or oral membrane) is first seen at the site of the future face, between the primordium of the heart and the rapidly enlarging primordium of the brain. It is composed of ectoderm externally and endoderm internally. It lies at the beginning of the digestive tract and breaks down during the 4th week in order to form the opening between the future oral cavity (primitive mouth or stomodeum) and the foregut. The oropharyngeal membrane breaks down when it stops growing. While tissues around it expand very rapidly, the oropharyngeal membrane’s non-proliferating cells are gradually pulled apart because they cannot fill the expanding area. The human face begins to form during the 4th week of embryonic development. By the 6th week the external face is completed. The development of the palate subdivides nasal and oral cavities. This development continues into the 12th week with completion of the soft palate. Human face during the 4th prenatal week. Around the centrally located oral pit are grouped the frontal and maxillary processes and the mandibular arch. 1 EMBRYOLOGY7 Dr.Ban A.Ghani Human face during the 5th prenatal week. The nasal pits develop and appear on the sides of the face. The frontal process now becomes the frontonasal process Human face during the 6th prenatal week. Nasal pits appear more centrally located in the medial nasal process. This is the result of growth of the lateral face, which also causes the eyes to approach the front of the face. -

Trávicí Systém
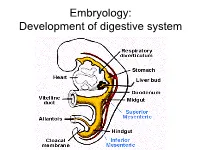
Embryology: Development of digestive system Embryo folding – incorporation of endoderm to form primitive gut. Outside of embryo – yolk sac and allantois. Vitelline duct Stomodeum (primitive mouth) the oral cavity + the salivary glands Proctodeum primitive anal pit Primitive gut whole digestive tube + accessory glands pharynx forgut midgut hindgut • The epithelium and glandular cells of associated glands of the gastrointestinal tract develop from endoderm • The connective tissue, muscle tissue and mesothelium are derived from splanchnic mesoderm • The enteric nervous system develops from neural crest primitive gut foregut midgut hindgut pharyngeal above ductus cloacal membrane omphalomesentericus membrane and yolk sack Derivatives of forgut – pharynx, esophagus (+ respiratory diverticul), stomach, cranial part of duodenum midgut – caudal part of duodenum (+ liver, gall bladder, pancreas), small intestine and part of large intestine (to the flexura coli sin.) hindgut – large intestine (from flexura coli sin.), rectum, upper part of anal canal Oral cavity • primitive mouth pit – stomodeum • lined with ectoderm • surrounded by: - processus frontalis (single) - proc. maxillares (paired) - proc. mandibulares (paired) • pharyngeal membrane (it ruptures during the 4th week, primitive gut communicates with amnionic cavity Pharyngeal (branchial) apparatus Pharyngeal arches • appear in weeks 4 - 5 • on the ventral side of the pharyngeal gut. • each arch has cartilage, cranial nerve, aortic arch artery and muscle • pharyngeal clefts and pouches -

A New Origin for the Maxillary Jaw
Developmental Biology 276 (2004) 207–224 www.elsevier.com/locate/ydbio A new origin for the maxillary jaw Sang-Hwy Leea, Olivier Be´dardb,1, Marcela Buchtova´b, Katherine Fub, Joy M. Richmanb,* aDepartment of Oral, Maxillofacial Surgery and Oral Science Research Center, Medical Science and Engineering Research Center, BK 21 Project for Medical Science, College of Dentistry Yonsei University, Seoul, Korea bDepartment of Oral Health Sciences, Faculty of Dentistry, University of British Columbia, Vancouver, BC, Canada, V6T 1Z3 Received for publication 7 April 2004, revised 5 August 2004, accepted 31 August 2004 Available online 5 October 2004 Abstract One conserved feature of craniofacial development is that the first pharyngeal arch has two components, the maxillary and mandibular, which then form the upper and lower jaws, respectively. However, until now, there have been no tests of whether the maxillary cells originate entirely within the first pharyngeal arch or whether they originate in a separate condensation, cranial to the first arch. We therefore constructed a fate map of the pharyngeal arches and environs with a series of dye injections into stage 13–17 chicken embryos. We found that from the earliest stage examined, the major contribution to the maxillary bud is from post-optic mesenchyme with a relatively minor contribution from the maxillo-mandibular cleft. Cells labeled within the first pharyngeal arch contributed exclusively to the mandibular prominence. Gene expression data showed that there were different molecular codes for the cranial and caudal maxillary prominence. Two of the genes examined, Rarb (retinoic acid receptor b) and Bmp4 (bone morphogenetic protein) were expressed in the post-optic mesenchyme and epithelium prior to formation of the maxillary prominence and then were restricted to the cranial half of the maxillary prominence. -

Índice De Denominacións Españolas
VOCABULARIO Índice de denominacións españolas 255 VOCABULARIO 256 VOCABULARIO agente tensioactivo pulmonar, 2441 A agranulocito, 32 abaxial, 3 agujero aórtico, 1317 abertura pupilar, 6 agujero de la vena cava, 1178 abierto de atrás, 4 agujero dental inferior, 1179 abierto de delante, 5 agujero magno, 1182 ablación, 1717 agujero mandibular, 1179 abomaso, 7 agujero mentoniano, 1180 acetábulo, 10 agujero obturado, 1181 ácido biliar, 11 agujero occipital, 1182 ácido desoxirribonucleico, 12 agujero oval, 1183 ácido desoxirribonucleico agujero sacro, 1184 nucleosómico, 28 agujero vertebral, 1185 ácido nucleico, 13 aire, 1560 ácido ribonucleico, 14 ala, 1 ácido ribonucleico mensajero, 167 ala de la nariz, 2 ácido ribonucleico ribosómico, 168 alantoamnios, 33 acino hepático, 15 alantoides, 34 acorne, 16 albardado, 35 acostarse, 850 albugínea, 2574 acromático, 17 aldosterona, 36 acromatina, 18 almohadilla, 38 acromion, 19 almohadilla carpiana, 39 acrosoma, 20 almohadilla córnea, 40 ACTH, 1335 almohadilla dental, 41 actina, 21 almohadilla dentaria, 41 actina F, 22 almohadilla digital, 42 actina G, 23 almohadilla metacarpiana, 43 actitud, 24 almohadilla metatarsiana, 44 acueducto cerebral, 25 almohadilla tarsiana, 45 acueducto de Silvio, 25 alocórtex, 46 acueducto mesencefálico, 25 alto de cola, 2260 adamantoblasto, 59 altura a la punta de la espalda, 56 adenohipófisis, 26 altura anterior de la espalda, 56 ADH, 1336 altura del esternón, 47 adipocito, 27 altura del pecho, 48 ADN, 12 altura del tórax, 48 ADN nucleosómico, 28 alunarado, 49 ADNn, 28 -
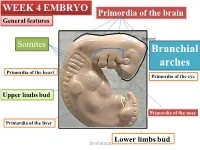
Cleft Palate Are Common Defects That Result in Abnormal Facial Appearance and Defective Speech
WEEK 4 EMBRYO Primordia of the brain General features shatarat Somites Branchial arches Primordia of the heart Primordia of the eye Upper limbs bud Primordia of the nose Primordia of the liver Lower limbs bud Dr.shatarat The most important feature in the development of the head and neck is the Formation of THE PHARYNGEAL OR BRANCHIALARCHES shatarat Dr.shatarat Is it branchial or is it pharyngeal arch? development of pharyngeal arches resembles formation of gills in fish However, in the human embryo real gills (branchia) are never formed. Therefore, the term pharyngeal arches has been adopted for the human embryo Dr.shatarat shatarat THE PHARYNGEALARCHES appear in the fourth and fifth weeks of development Dr.shatarat Why the appear? Migration of cells from epiblast Dr.shatarat 1-PARAXIALMESODERM 2-LATERALPLATE MESODERM 3-NEURALCREST Dr.shatarat Migration of the cells from the occipital Myotomes into the future mouth to form the tongue Occipital somites This is an explanation to how the arches appear…. as a result of migration of the cells from the medial mesoderm (somites) into tongue the regions of the future head and neck. As we mentioned there are other reasons Dr.shatarat In a cross section of the embryo in the area of the head and neck The following can be noticed THE PHARYNGEALARCHES shatarat THE PHARYNGEALARCHES are separated by deep clefts known as PHARYNGEAL CLEFTS with development of the arches and clefts, a number of outpocketings, The pharyngeal pouches appear Dr.shatarat 1-PHARYNGEAL ARCHs Dr.shatarat 6 However, The fifth and sixth arches are rudimentary and are not visible on the surface of the embryo T h e y a re n u m b e r e d i n c r a n i o ca ud a During the fifth week, the second pharyngeal l s e arch enlarges and overgrows the third and qu fourth arches, forming the ectodermal e n c depression called cervical sinus e Dr.shatarat Each pharyngeal arch consists of: 1-surface ECTODERM 2-a core of MESENCHYMALtissue 3- epithelium of ENDODERMAL origin Each pharyngeal arch contains: 1- An artery that arises from the primordial heart A. -
Ectodermal Pharyngeal Clefts
Embryology: Development of digestive system Flecture of embryo – incorporation of endoderm to form primitive gut. Outside of embryo – yolk sac and allantois. Vitelline duct Stomodeum (primitive mouth) the oral cavity + the salivary glands Proctodeum primitive anal pit Primitive gut whole digestive tube + accessory glands orofacial membrane Tracheo- esophageal septum Proctodeum cloacal membrane Primitive gut pharynx forgut midgut hindgut Derivatives of foregut – pharynx, (+ respiratory diverticle),esophagus, stomach, cranial part of duodenum, (+ liver, gall bladder pancreas) midgut – caudal part of duodenum, small intestine and part of large intestine (1/3 of colon transv.) hindgut – the rest of large intestine, rectum, upper part of the anal canal Tissues in GIT • The epithelium of gut and glandular cells of associated glands of the gastrointestinal tract develop from endoderm • The connective tissue, muscle tissue and mesothelium derive from splanchnic mesoderm • The enteric nervous system develops from neural crest primitive gut foregut midgut hindgut from above ductus to cloacal pharyngeal omphalomesentericus membrane membrane and yolk sack Pharyngeal membrane liver, bile duct, pancreas Rectum Origin: forgut, midgut. hindgut Oral cavity • primitive mouth pit – stomodeum • lined with ectoderm • surrounded by: - processus frontalis (single) - proc. maxillares (paired) - proc. mandibulares (paired) • orophacial membrane (it ruptures during the 4th week, primitive gut communicates with amnionic cavity) Pharyngeal (branchial) apparatus