A New Origin for the Maxillary Jaw
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Te2, Part Iii
TERMINOLOGIA EMBRYOLOGICA Second Edition International Embryological Terminology FIPAT The Federative International Programme for Anatomical Terminology A programme of the International Federation of Associations of Anatomists (IFAA) TE2, PART III Contents Caput V: Organogenesis Chapter 5: Organogenesis (continued) Systema respiratorium Respiratory system Systema urinarium Urinary system Systemata genitalia Genital systems Coeloma Coelom Glandulae endocrinae Endocrine glands Systema cardiovasculare Cardiovascular system Systema lymphoideum Lymphoid system Bibliographic Reference Citation: FIPAT. Terminologia Embryologica. 2nd ed. FIPAT.library.dal.ca. Federative International Programme for Anatomical Terminology, February 2017 Published pending approval by the General Assembly at the next Congress of IFAA (2019) Creative Commons License: The publication of Terminologia Embryologica is under a Creative Commons Attribution-NoDerivatives 4.0 International (CC BY-ND 4.0) license The individual terms in this terminology are within the public domain. Statements about terms being part of this international standard terminology should use the above bibliographic reference to cite this terminology. The unaltered PDF files of this terminology may be freely copied and distributed by users. IFAA member societies are authorized to publish translations of this terminology. Authors of other works that might be considered derivative should write to the Chair of FIPAT for permission to publish a derivative work. Caput V: ORGANOGENESIS Chapter 5: ORGANOGENESIS -

Induction and Specification of Cranial Placodes ⁎ Gerhard Schlosser
Developmental Biology 294 (2006) 303–351 www.elsevier.com/locate/ydbio Review Induction and specification of cranial placodes ⁎ Gerhard Schlosser Brain Research Institute, AG Roth, University of Bremen, FB2, PO Box 330440, 28334 Bremen, Germany Received for publication 6 October 2005; revised 22 December 2005; accepted 23 December 2005 Available online 3 May 2006 Abstract Cranial placodes are specialized regions of the ectoderm, which give rise to various sensory ganglia and contribute to the pituitary gland and sensory organs of the vertebrate head. They include the adenohypophyseal, olfactory, lens, trigeminal, and profundal placodes, a series of epibranchial placodes, an otic placode, and a series of lateral line placodes. After a long period of neglect, recent years have seen a resurgence of interest in placode induction and specification. There is increasing evidence that all placodes despite their different developmental fates originate from a common panplacodal primordium around the neural plate. This common primordium is defined by the expression of transcription factors of the Six1/2, Six4/5, and Eya families, which later continue to be expressed in all placodes and appear to promote generic placodal properties such as proliferation, the capacity for morphogenetic movements, and neuronal differentiation. A large number of other transcription factors are expressed in subdomains of the panplacodal primordium and appear to contribute to the specification of particular subsets of placodes. This review first provides a brief overview of different cranial placodes and then synthesizes evidence for the common origin of all placodes from a panplacodal primordium. The role of various transcription factors for the development of the different placodes is addressed next, and it is discussed how individual placodes may be specified and compartmentalized within the panplacodal primordium. -

Endothelium in the Pharyngeal Arches 3, 4 and 6 Is Derived from the Second Heart Field
Thomas Jefferson University Jefferson Digital Commons Center for Translational Medicine Faculty Papers Center for Translational Medicine 1-15-2017 Endothelium in the pharyngeal arches 3, 4 and 6 is derived from the second heart field. Xia Wang Thomas Jefferson University Dongying Chen Thomas Jefferson University Kelley Chen Thomas Jefferson University Ali Jubran Thomas Jefferson University AnnJosette Ramirez Thomas Jefferson University Follow this and additional works at: https://jdc.jefferson.edu/transmedfp Part of the Translational Medical Research Commons LetSee next us page know for additional how authors access to this document benefits ouy Recommended Citation Wang, Xia; Chen, Dongying; Chen, Kelley; Jubran, Ali; Ramirez, AnnJosette; and Astrof, Sophie, "Endothelium in the pharyngeal arches 3, 4 and 6 is derived from the second heart field." (2017). Center for Translational Medicine Faculty Papers. Paper 45. https://jdc.jefferson.edu/transmedfp/45 This Article is brought to you for free and open access by the Jefferson Digital Commons. The Jefferson Digital Commons is a service of Thomas Jefferson University's Center for Teaching and Learning (CTL). The Commons is a showcase for Jefferson books and journals, peer-reviewed scholarly publications, unique historical collections from the University archives, and teaching tools. The Jefferson Digital Commons allows researchers and interested readers anywhere in the world to learn about and keep up to date with Jefferson scholarship. This article has been accepted for inclusion in Center for Translational Medicine Faculty Papers by an authorized administrator of the Jefferson Digital Commons. For more information, please contact: [email protected]. Authors Xia Wang, Dongying Chen, Kelley Chen, Ali Jubran, AnnJosette Ramirez, and Sophie Astrof This article is available at Jefferson Digital Commons: https://jdc.jefferson.edu/transmedfp/45 HHS Public Access Author manuscript Author ManuscriptAuthor Manuscript Author Dev Biol Manuscript Author . -
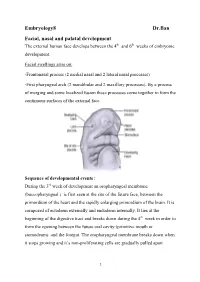
Embryology8 Dr.Ban Facial, Nasal and Palatal Development the External Human Face Develops Between the 4Th and 6Th Weeks of Embryonic Development
Embryology8 Dr.Ban Facial, nasal and palatal development The external human face develops between the 4th and 6th weeks of embryonic development. Facial swellings arise on: -Frontonasal process (2 medial nasal and 2 lateral nasal processes) -First pharyngeal arch (2 mandibular and 2 maxillary processes). By a process of merging and some localized fusion these processes come together to form the continuous surfaces of the external face. Sequence of developmental events : During the 3rd week of development an oropharyngeal membrane (buccopharyngeal ) is first seen at the site of the future face, between the primordium of the heart and the rapidly enlarging primordium of the brain. It is composed of ectoderm externally and endoderm internally. It lies at the beginning of the digestive tract and breaks down during the 4th week in order to form the opening between the future oral cavity (primitive mouth or stomodeum) and the foregut. The oropharyngeal membrane breaks down when it stops growing and it’s non-proliferating cells are gradually pulled apart 1 because they cannot fill the expanding area.The tissues around it expand very rapidly. The face develops from five primordia that appear in the fourth week: the frontonasal prominence, the two maxillary swellings, and the two mandibular swellings. The external face forms from two sources that surround the oropharyngeal membrane 1-Tissues of the frontonasal process that cover the forebrain, predominantly of neural crest origin. 2-The tissues of the first (or mandibular) pharyngeal arch, of mixed mesoderm and neural crest origin Face initially formed by 5 mesenchymal swellings ( prominences): Two mandibular prominences Two maxillary prominences Frontonasal prominence (midline structure is a single structure that is ventral to the forebrain. -

Vocabulario De Morfoloxía, Anatomía E Citoloxía Veterinaria
Vocabulario de Morfoloxía, anatomía e citoloxía veterinaria (galego-español-inglés) Servizo de Normalización Lingüística Universidade de Santiago de Compostela COLECCIÓN VOCABULARIOS TEMÁTICOS N.º 4 SERVIZO DE NORMALIZACIÓN LINGÜÍSTICA Vocabulario de Morfoloxía, anatomía e citoloxía veterinaria (galego-español-inglés) 2008 UNIVERSIDADE DE SANTIAGO DE COMPOSTELA VOCABULARIO de morfoloxía, anatomía e citoloxía veterinaria : (galego-español- inglés) / coordinador Xusto A. Rodríguez Río, Servizo de Normalización Lingüística ; autores Matilde Lombardero Fernández ... [et al.]. – Santiago de Compostela : Universidade de Santiago de Compostela, Servizo de Publicacións e Intercambio Científico, 2008. – 369 p. ; 21 cm. – (Vocabularios temáticos ; 4). - D.L. C 2458-2008. – ISBN 978-84-9887-018-3 1.Medicina �������������������������������������������������������������������������veterinaria-Diccionarios�������������������������������������������������. 2.Galego (Lingua)-Glosarios, vocabularios, etc. políglotas. I.Lombardero Fernández, Matilde. II.Rodríguez Rio, Xusto A. coord. III. Universidade de Santiago de Compostela. Servizo de Normalización Lingüística, coord. IV.Universidade de Santiago de Compostela. Servizo de Publicacións e Intercambio Científico, ed. V.Serie. 591.4(038)=699=60=20 Coordinador Xusto A. Rodríguez Río (Área de Terminoloxía. Servizo de Normalización Lingüística. Universidade de Santiago de Compostela) Autoras/res Matilde Lombardero Fernández (doutora en Veterinaria e profesora do Departamento de Anatomía e Produción Animal. -

D Inosaur Paleobiology
Topics in Paleobiology The study of dinosaurs has been experiencing a remarkable renaissance over the past few decades. Scientifi c understanding of dinosaur anatomy, biology, and evolution has advanced to such a degree that paleontologists often know more about 100-million-year-old dinosaurs than many species of living organisms. This book provides a contemporary review of dinosaur science intended for students, researchers, and dinosaur enthusiasts. It reviews the latest knowledge on dinosaur anatomy and phylogeny, Brusatte how dinosaurs functioned as living animals, and the grand narrative of dinosaur evolution across the Mesozoic. A particular focus is on the fossil evidence and explicit methods that allow paleontologists to study dinosaurs in rigorous detail. Scientifi c knowledge of dinosaur biology and evolution is shifting fast, Dinosaur and this book aims to summarize current understanding of dinosaur science in a technical, but accessible, style, supplemented with vivid photographs and illustrations. Paleobiology Dinosaur The Topics in Paleobiology Series is published in collaboration with the Palaeontological Association, Paleobiology and is edited by Professor Mike Benton, University of Bristol. Stephen Brusatte is a vertebrate paleontologist and PhD student at Columbia University and the American Museum of Natural History. His research focuses on the anatomy, systematics, and evolution of fossil vertebrates, especially theropod dinosaurs. He is particularly interested in the origin of major groups such Stephen L. Brusatte as dinosaurs, birds, and mammals. Steve is the author of over 40 research papers and three books, and his work has been profi led in The New York Times, on BBC Television and NPR, and in many other press outlets. -

Pharyngeal Arch Cheat Sheet V3
Pharyngeal Arches v3 (updates in red) Each pharyngeal arch contains: (1) a skeletal component (early on, a cartilaginous rod); (2) a muscular component; (3) an aortic arch; and (4) a nerve. Pharyngeal Arch Skeletal Component Muscles Aortic Arch Nerve First P. Arch Meckel's cartilage (malleus, incus) mm. of mastication, ant. belly 1st aortic arch Trigeminal N “mandibular maxillary prominence (maxilla*) of digastric, mylohyoid, tensor (maxillary AA) V2 and V3 arch” mandibular prominence (mandible*) tympani, tensor veli palatini Second P. Arch Reichert's cartilage (stapes, styloid mm. of facial expression, 2nd aortic arch Facial N “hyoid arch” process, stylohyoid lig.) buccinator, platysma, stapedius, (stapedial AA) VII hyoid, lesser cornua & superior body stylohyoid, post. belly of digastric Third P. Arch hyoid, greater cornua & inferior body stylopharyngeus 3rd aortic arch Glossophar. N (common carotids) IX Fourth P. Arch thyroid cartilage, epiglottic cartilage† cricothyroid, all intrinsic mm. 4th aortic arch Vagus: SLN of soft palate except tensor (aorta, R subclav.‡) & pharyn. plex. veli palatini X Fifth P. Arch - no derivatives - Sixth P. Arch cricoid, arytenoid, and corniculate all intrinsic mm. of larynx 6th aortic arch Vagus: RLN cartilages† except cricothyroid (pulmonary AA, X ductus arteriosus) * The maxillary and mandibular prominences are modeled on the first arch, but the bones develop separately from the first arch cartilage. † According to some sources the corniculate and cuneiform cartilages are derived from the 4th arch, not the 6th; I may revise this later on. ‡ Right subclavian A is derived from 4th aortic arch, right dorsal aorta, and right 7th intersegmental A; left subclavian A is derived from left 7th intersegmental A only, with no aortic arch component. -

Anatomical Basis of Craniofacial Birth Defects
AnatomicalAnatomical BasisBasis ofof CraniofacialCraniofacial BirthBirth DefectsDefects Handout download: http://www.oucom.ohiou.edu/dbms-witmer/peds-rpac.htm LawrenceLawrence M.M. Witmer,Witmer, PhDPhD Department of Biomedical Sciences College of Osteopathic Medicine Ohio University Athens, Ohio 45701 [email protected] 18 October 2000 DevelopmentDevelopment ofof thethe FaceFace II • 5 facial primordia • Frontonasal prominence • Paired maxillary prominences • Paired mandibular prominences • Surround primordial mouth (stomodeum) • Neural crest: source for almost all connective tissues in the face • Frontonasal prominence forms forehead and nose and a short margin of mouth • Lower jaw and lip form first • Nasal placodes (and pit): surrounded by medial & lateral nasal prominences • Nasal pit remains connected to mouth • Maxillary prominences grow toward each other, pushing nasal prominences medially From Moore 1982 DevelopmentDevelopment ofof thethe FaceFace IIII • Medial nasal prominences merge with each other and with lateral nasal & maxillary prominences • Nasolacrimal groove: between lateral nasal and maxillary prominences • Becomes nasolacrimal duct • Duct forms as solid epithelial cord that later canalizes • Nasolacrimal duct atresia • Failure to completely canalize • 6% of newborns • Intermaxillary segment • Merger of medial nasal prominences • Gives rise to philtrum, premaxillary bones, primary palate From Moore 1982 SummarySummary ofof FacialFacial DevelopmentDevelopment From Moore 1982 Disruptions in the formation -

Biology of the Rabbit
Journal of the American Association for Laboratory Animal Science Vol 45, No 1 Copyright 2006 January 2006 by the American Association for Laboratory Animal Science Pages 8–24 Historical Special Topic Overview on Rabbit Comparative Biology Biology of the Rabbit Nathan R. Brewer Editor’s note: In recognition of Dr. Nathan Brewer’s many years of dedicated service to AALAS and the community of research animal care specialists, the premier issue of JAALAS includes the following compilation of Dr. Brewer’s essays on rabbit anatomy and physiology. These essays were originally published in the ASLAP newsletter (formerly called Synapse), and are reprinted here with the permission and endorsement of that organization. I would like to thank Nina Hahn, Jane Lacher, and Nancy Austin for assistance in compiling these essays. Publishing this information in JAALAS allows Dr. Brewer’s work to become part of the searchable literature for laboratory animal science and medicine and also assures that the literature references and information he compiled will not be lost to posterity. However, readers should note that this material has undergone only minor editing for style, has not been edited for content, and, most importantly, has not undergone peer review. With the agreement of the associate editors and the AALAS leadership, I elected to forego peer review of this work, in contradiction to standard JAALAS policy, based on the status of this material as pre-published information from an affiliate organization that holds the copyright and on the esteem in which we hold for Dr. Brewer as a founding father of our organization. -

Comparison of Greater Palatine Nerve Block with Intravenous Fentanyl for Postoperative Analgesia Following Palatoplasty in Children
Jemds.com Original Research Article Comparison of Greater Palatine Nerve Block with Intravenous Fentanyl for Postoperative Analgesia Following Palatoplasty in Children Amol Singam1, Saranya Rallabhandi2, Tapan Dhumey3 1Department of Anaesthesiology, JNMC, DMIMS, Sawangi Meghe, Wardha Maharashtra, India. 2Department of Anaesthesiology, JNMC, DMIMS, Sawangi Meghe, Wardha, Maharashtra, India. 3Department of Anaesthesiology, JNMC, DMIMS, Sawangi Meghe, Wardha, Maharashtra, India. ABSTRACT BACKGROUND Good pain relief after palatoplasty is important as inadequate analgesia with vigorous Corresponding Author: cry leads to wound dehiscence, removal of sutures and extra nursing care. Decrease Dr. Saranya Rallabhandi, in oxygen requirement and cardio-respiratory demand occur with good pain relief Assisstant Professor, and also promotes early recovery. Preoperative opioids have concerns like sedation, Department of Anesthesiology, AVBRH, DMIMS (DU), Sawangi Meghe, respiratory depression and airway compromise. Greater palatine nerve block with Wardha- 442001, Maharashtra, India. bupivacaine is safe and effective without the risk of respiratory depression. The study E-mail: [email protected] was done to compare pain relief postoperatively with intravenous fentanyl and greater palatine nerve block in children following palatoplasty. DOI: 10.14260/jemds/2020/549 METHODS How to Cite This Article: 80 children of ASA I & II, between 1 to 7 years were included and allocated into two Singam A, Rallabhandi S, Dhumey T. Comparison of greater palatine nerve block groups of 40 each. Analgesic medication was given preoperatively after induction of with intravenous fentanyl for postoperative general anaesthesia, children in Group B received greater palatine nerve block with analgesia following palatoplasty in -1 2 mL 0.25% inj. Bupivacaine (1 mL on each side) and Group F received 2 μg Kg I.V. -

Development and Malformations of Face and Palate
Development and malformations of face and palate Compiled by András Csillag and Andrea D. Székely GERMINAL LAYER DERIVATIVES ECTODERM contributing to the formation of the face appears by the 4th week. The oropharyngeal membrane (interface between ECTODERM and ENDODERM) is located in front of the later palatine tonsils. Ectodermal structures limiting the stomodeum participate in the formation of the face, as well as of the nasal and oral cavities. MESENCHYME that fills the pharyngeal arches derives from the neural crest ECTOMESENCHYME DEVELOPMENT OF THE FACE WEEK 6 The face is formed by 5 processes approximately 24 days Frontonasal prominence (1) Maxillary prominence (2) 1st arch Mandibular prominence (2) nasal (olfactory) pits form surrounded by the medial and lateral nasal processes nasolacrimal groove separates the lateral nasal process from the maxillary process maxillary processes fuse with the medial nasal processes lateral nasal processes fuse with the maxillary processes, thus obliterating the nasolacrimal groove. DEVELOPMENT OF THE FACE 5-week embryo 6-week embryo 7-week embryo. 10-week embryo The nasal prominences are gradually Maxillary prominences have fused separated from the maxillary with the medial nasal prominences. prominence by deep furrows. CEPHALIC PRIMORDIA - PLACODES Olfactory placode Pharyngeal Stomodeum” arches Nasal placodes - thickenings of the surface ectoderm (later differentiate into the olfactory epithelium) DEVELOPMENT OF THE NASAL CAVITIES AND THE HARD PALATE By week 5 the placodes form the nasal pits. They further invaginate and the pits approach the primitive oral cavity. A thin oronasal membrane separates the two cavities. By its rupture the primitive choanae will form DEVELOPMENT OF THE NASAL CAVITIES AND THE HARD PALATE By week 8, a partition forms between the primitive nasal chambers and the oral cavity The primary palate - the anterior aspect derives from the intermaxillary segment (or median palatine process, formed by the medial nasal processes). -
Lab Manual Axial Skeleton Atla
1 PRE-LAB EXERCISES When studying the skeletal system, the bones are often sorted into two broad categories: the axial skeleton and the appendicular skeleton. This lab focuses on the axial skeleton, which consists of the bones that form the axis of the body. The axial skeleton includes bones in the skull, vertebrae, and thoracic cage, as well as the auditory ossicles and hyoid bone. In addition to learning about all the bones of the axial skeleton, it is also important to identify some significant bone markings. Bone markings can have many shapes, including holes, round or sharp projections, and shallow or deep valleys, among others. These markings on the bones serve many purposes, including forming attachments to other bones or muscles and allowing passage of a blood vessel or nerve. It is helpful to understand the meanings of some of the more common bone marking terms. Before we get started, look up the definitions of these common bone marking terms: Canal: Condyle: Facet: Fissure: Foramen: (see Module 10.18 Foramina of Skull) Fossa: Margin: Process: Throughout this exercise, you will notice bold terms. This is meant to focus your attention on these important words. Make sure you pay attention to any bold words and know how to explain their definitions and/or where they are located. Use the following modules to guide your exploration of the axial skeleton. As you explore these bones in Visible Body’s app, also locate the bones and bone markings on any available charts, models, or specimens. You may also find it helpful to palpate bones on yourself or make drawings of the bones with the bone markings labeled.