Characterization of Epithelial Domains in The
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Te2, Part Iii
TERMINOLOGIA EMBRYOLOGICA Second Edition International Embryological Terminology FIPAT The Federative International Programme for Anatomical Terminology A programme of the International Federation of Associations of Anatomists (IFAA) TE2, PART III Contents Caput V: Organogenesis Chapter 5: Organogenesis (continued) Systema respiratorium Respiratory system Systema urinarium Urinary system Systemata genitalia Genital systems Coeloma Coelom Glandulae endocrinae Endocrine glands Systema cardiovasculare Cardiovascular system Systema lymphoideum Lymphoid system Bibliographic Reference Citation: FIPAT. Terminologia Embryologica. 2nd ed. FIPAT.library.dal.ca. Federative International Programme for Anatomical Terminology, February 2017 Published pending approval by the General Assembly at the next Congress of IFAA (2019) Creative Commons License: The publication of Terminologia Embryologica is under a Creative Commons Attribution-NoDerivatives 4.0 International (CC BY-ND 4.0) license The individual terms in this terminology are within the public domain. Statements about terms being part of this international standard terminology should use the above bibliographic reference to cite this terminology. The unaltered PDF files of this terminology may be freely copied and distributed by users. IFAA member societies are authorized to publish translations of this terminology. Authors of other works that might be considered derivative should write to the Chair of FIPAT for permission to publish a derivative work. Caput V: ORGANOGENESIS Chapter 5: ORGANOGENESIS -

Induction and Specification of Cranial Placodes ⁎ Gerhard Schlosser
Developmental Biology 294 (2006) 303–351 www.elsevier.com/locate/ydbio Review Induction and specification of cranial placodes ⁎ Gerhard Schlosser Brain Research Institute, AG Roth, University of Bremen, FB2, PO Box 330440, 28334 Bremen, Germany Received for publication 6 October 2005; revised 22 December 2005; accepted 23 December 2005 Available online 3 May 2006 Abstract Cranial placodes are specialized regions of the ectoderm, which give rise to various sensory ganglia and contribute to the pituitary gland and sensory organs of the vertebrate head. They include the adenohypophyseal, olfactory, lens, trigeminal, and profundal placodes, a series of epibranchial placodes, an otic placode, and a series of lateral line placodes. After a long period of neglect, recent years have seen a resurgence of interest in placode induction and specification. There is increasing evidence that all placodes despite their different developmental fates originate from a common panplacodal primordium around the neural plate. This common primordium is defined by the expression of transcription factors of the Six1/2, Six4/5, and Eya families, which later continue to be expressed in all placodes and appear to promote generic placodal properties such as proliferation, the capacity for morphogenetic movements, and neuronal differentiation. A large number of other transcription factors are expressed in subdomains of the panplacodal primordium and appear to contribute to the specification of particular subsets of placodes. This review first provides a brief overview of different cranial placodes and then synthesizes evidence for the common origin of all placodes from a panplacodal primordium. The role of various transcription factors for the development of the different placodes is addressed next, and it is discussed how individual placodes may be specified and compartmentalized within the panplacodal primordium. -

Vocabulario De Morfoloxía, Anatomía E Citoloxía Veterinaria
Vocabulario de Morfoloxía, anatomía e citoloxía veterinaria (galego-español-inglés) Servizo de Normalización Lingüística Universidade de Santiago de Compostela COLECCIÓN VOCABULARIOS TEMÁTICOS N.º 4 SERVIZO DE NORMALIZACIÓN LINGÜÍSTICA Vocabulario de Morfoloxía, anatomía e citoloxía veterinaria (galego-español-inglés) 2008 UNIVERSIDADE DE SANTIAGO DE COMPOSTELA VOCABULARIO de morfoloxía, anatomía e citoloxía veterinaria : (galego-español- inglés) / coordinador Xusto A. Rodríguez Río, Servizo de Normalización Lingüística ; autores Matilde Lombardero Fernández ... [et al.]. – Santiago de Compostela : Universidade de Santiago de Compostela, Servizo de Publicacións e Intercambio Científico, 2008. – 369 p. ; 21 cm. – (Vocabularios temáticos ; 4). - D.L. C 2458-2008. – ISBN 978-84-9887-018-3 1.Medicina �������������������������������������������������������������������������veterinaria-Diccionarios�������������������������������������������������. 2.Galego (Lingua)-Glosarios, vocabularios, etc. políglotas. I.Lombardero Fernández, Matilde. II.Rodríguez Rio, Xusto A. coord. III. Universidade de Santiago de Compostela. Servizo de Normalización Lingüística, coord. IV.Universidade de Santiago de Compostela. Servizo de Publicacións e Intercambio Científico, ed. V.Serie. 591.4(038)=699=60=20 Coordinador Xusto A. Rodríguez Río (Área de Terminoloxía. Servizo de Normalización Lingüística. Universidade de Santiago de Compostela) Autoras/res Matilde Lombardero Fernández (doutora en Veterinaria e profesora do Departamento de Anatomía e Produción Animal. -

Loss-Of-Function Mutation in the Prokineticin 2 Gene Causes
Loss-of-function mutation in the prokineticin 2 gene SEE COMMENTARY causes Kallmann syndrome and normosmic idiopathic hypogonadotropic hypogonadism Nelly Pitteloud*†, Chengkang Zhang‡, Duarte Pignatelli§, Jia-Da Li‡, Taneli Raivio*, Lindsay W. Cole*, Lacey Plummer*, Elka E. Jacobson-Dickman*, Pamela L. Mellon¶, Qun-Yong Zhou‡, and William F. Crowley, Jr.* *Reproductive Endocrine Unit, Department of Medicine and Harvard Reproductive Endocrine Science Centers, Massachusetts General Hospital, Boston, MA 02114; ‡Department of Pharmacology, University of California, Irvine, CA 92697; §Department of Endocrinology, Laboratory of Cellular and Molecular Biology, Institute of Molecular Pathology and Immunology, University of Porto, San Joa˜o Hospital, 4200-465 Porto, Portugal; and ¶Departments of Reproductive Medicine and Neurosciences, University of California at San Diego, La Jolla, CA 92093 Communicated by Patricia K. Donahoe, Massachusetts General Hospital, Boston, MA, August 14, 2007 (received for review May 8, 2007) Gonadotropin-releasing hormone (GnRH) deficiency in the human associated with KS, although no functional data on the mutant presents either as normosmic idiopathic hypogonadotropic hypo- proteins were provided (17). Herein, we demonstrate that homozy- gonadism (nIHH) or with anosmia [Kallmann syndrome (KS)]. To gous loss-of-function mutations in the PROK2 gene cause IHH in date, several loci have been identified to cause these disorders, but mice and humans. only 30% of cases exhibit mutations in known genes. Recently, murine studies have demonstrated a critical role of the prokineticin Results pathway in olfactory bulb morphogenesis and GnRH secretion. Molecular Analysis of PROK2 Gene. A homozygous single base pair Therefore, we hypothesize that mutations in prokineticin 2 deletion in exon 2 of the PROK2 gene (c.[163delA]ϩ [163delA]) (PROK2) underlie some cases of KS in humans and that animals was identified in the proband, in his brother with KS, and in his deficient in Prok2 would be hypogonadotropic. -

A Combined Approach of Teaching Head Development Using Embryology and Comparative Anatomy
Edorium J Anat Embryo 2016;3:17–27. Danowitz et al. 17 www.edoriumjournals.com/ej/ae REVIEW ARTICLE PEER REVIEWED | OPEN ACCESS A combined approach of teaching head development using embryology and comparative anatomy Melinda Danowitz, Hong Zheng, Adriana Guigova, Nikos Solounias ABSTRACT the evolutionary changes of many structures allows for a greater understanding of the Many aspects of human head embryology reflect human embryology, and removes the need for its evolutionary development. The pharyngeal memorization of seemingly complex processes. arches, a major component of head development, A link to comparative evolutionary anatomy originally functioned in filter feeding and provides context to the purpose and morphology vascular exchange, which is why each arch of primitive structures, and clarifies several has associated vasculature and muscles. The issues in human head development. primitive tongue had few-associated muscles and was responsible for simple movements; the Keywords: Anatomy education, Embryology, Head human tongue evolved post-otic somites that and neck, Pharyngeal arches migrate to the tongue and develop the majority of the tongue musculature. These somites originate How to cite this article outside the tongue, and the motor innervation therefore differs from the general and special Danowitz M, Zheng H, Guigova A, Solounias N. A sensory innervation. In the primitive condition, combined approach of teaching head development the trapezius and sternocleidomastoid belonged using embryology and comparative anatomy. to a single muscle group that were involved in Edorium J Anat Embryo 2016;3:17–27. gill movements; they separate into two muscles with the reduction of certain skeletal elements, but retain the same innervation. -

Congenital Anomalies of the Nose
133 Congenital Anomalies of the Nose Jamie L. Funamura, MD1 Travis T. Tollefson, MD, MPH, FACS2 1 Department of Otolaryngology and Communication Enhancement, Address for correspondence Travis T. Tollefson, MD, MPH, FACS, Facial Children’s Hospital Boston, Boston, Massachusetts Plastic and Reconstructive Surgery, Department of Otolaryngology- 2 Department of Otolaryngology, University of California, Davis, Head and Neck Surgery, University of California, Davis, 2521 Stockton Sacramento, California Blvd., Suite 7200, Sacramento, CA 95817 (e-mail: [email protected]). Facial Plast Surg 2016;32:133–141. Abstract Congenital anomalies of the nose range from complete aplasia of the nose to duplications and nasal masses. Nasal development is the result of a complex embryo- logic patterning and fusion of multiple primordial structures. Loss of signaling proteins or failure of migration or proliferation can result in structural anomalies with significant Keywords cosmetic and functional consequences. Congenital anomalies of the nose can be ► nasal deformities categorized into four broad categories: (1) aplastic or hypoplastic, (2) hyperplastic or ► nasal dermoid duplications, (3) clefts, and (4) nasal masses. Our knowledge of the embryologic origin ► Tessier cleft of these anomalies helps dictate subsequent work-up for associated conditions, and the ► nasal cleft appropriate treatment or surgical approach to manage newborns and children with ► nasal hemangioma these anomalies. – Congenital anomalies of the nose are thought to be relatively side1 4 (►Fig. 1A, B). The medial processes will ultimately fuse, rare, affecting approximately 1 in every 20,000 to 40,000 live contributing to the nasal septum and the medial crura of the births.1 The exact incidence is difficult to quantify, as minor lower lateral cartilages. -

Evolution of the Nasal Structure in the Lower Tetrapods
AM. ZOOLOCIST, 7:397-413 (1967). Evolution of the Nasal Structure in the Lower Tetrapods THOMAS S. PARSONS Department of Zoology, University of Toronto, Toronto, Ontario, Canada SYNOPSIS. The gross structure of the nasal cavities and the distribution of the various types of epithelium lining them are described briefly; each living order of amphibians and reptiles possesses a characteristic and distinctive pattern. In most groups there are two sensory areas, one lined by olfactory epithelium with nerve libers leading to the main olfactory bulb and the other by vomeronasal epithelium Downloaded from https://academic.oup.com/icb/article/7/3/397/244929 by guest on 04 October 2021 with fibers to the accessory bulb. All amniotes except turtles have the vomeronasal epithelium in a ventromedial outpocketing of the nose, the Jacobson's organ, and have one or more conchae projecting into the nasal cavity from the lateral wall. Although urodeles and turtles possess the simplest nasal structure, it is not possible to show that they are primitive or to define a basic pattern for either amphibians or reptiles; all the living orders are specialized and the nasal anatomy of extinct orders is unknown. Thus it is impossible, at present, to give a convincing picture of the course of nasal evolution in the lower tetrapods. Despite the rather optimistic title of this (1948, squamates), Stebbins (1948, squa- paper, I shall, unfortunately, be able to do mates), Bellairs and Boyd (1950, squa- iittle more than make a few guesses about mates), and Parsons (1959a, reptiles). Most the evolution of the nose. I can and will of the following descriptions are based on mention briefly the major features of the these works, although others, specifically nasal anatomy of the living orders of cited in various places, were also used. -

A New Origin for the Maxillary Jaw
Developmental Biology 276 (2004) 207–224 www.elsevier.com/locate/ydbio A new origin for the maxillary jaw Sang-Hwy Leea, Olivier Be´dardb,1, Marcela Buchtova´b, Katherine Fub, Joy M. Richmanb,* aDepartment of Oral, Maxillofacial Surgery and Oral Science Research Center, Medical Science and Engineering Research Center, BK 21 Project for Medical Science, College of Dentistry Yonsei University, Seoul, Korea bDepartment of Oral Health Sciences, Faculty of Dentistry, University of British Columbia, Vancouver, BC, Canada, V6T 1Z3 Received for publication 7 April 2004, revised 5 August 2004, accepted 31 August 2004 Available online 5 October 2004 Abstract One conserved feature of craniofacial development is that the first pharyngeal arch has two components, the maxillary and mandibular, which then form the upper and lower jaws, respectively. However, until now, there have been no tests of whether the maxillary cells originate entirely within the first pharyngeal arch or whether they originate in a separate condensation, cranial to the first arch. We therefore constructed a fate map of the pharyngeal arches and environs with a series of dye injections into stage 13–17 chicken embryos. We found that from the earliest stage examined, the major contribution to the maxillary bud is from post-optic mesenchyme with a relatively minor contribution from the maxillo-mandibular cleft. Cells labeled within the first pharyngeal arch contributed exclusively to the mandibular prominence. Gene expression data showed that there were different molecular codes for the cranial and caudal maxillary prominence. Two of the genes examined, Rarb (retinoic acid receptor b) and Bmp4 (bone morphogenetic protein) were expressed in the post-optic mesenchyme and epithelium prior to formation of the maxillary prominence and then were restricted to the cranial half of the maxillary prominence. -

Índice De Denominacións Españolas
VOCABULARIO Índice de denominacións españolas 255 VOCABULARIO 256 VOCABULARIO agente tensioactivo pulmonar, 2441 A agranulocito, 32 abaxial, 3 agujero aórtico, 1317 abertura pupilar, 6 agujero de la vena cava, 1178 abierto de atrás, 4 agujero dental inferior, 1179 abierto de delante, 5 agujero magno, 1182 ablación, 1717 agujero mandibular, 1179 abomaso, 7 agujero mentoniano, 1180 acetábulo, 10 agujero obturado, 1181 ácido biliar, 11 agujero occipital, 1182 ácido desoxirribonucleico, 12 agujero oval, 1183 ácido desoxirribonucleico agujero sacro, 1184 nucleosómico, 28 agujero vertebral, 1185 ácido nucleico, 13 aire, 1560 ácido ribonucleico, 14 ala, 1 ácido ribonucleico mensajero, 167 ala de la nariz, 2 ácido ribonucleico ribosómico, 168 alantoamnios, 33 acino hepático, 15 alantoides, 34 acorne, 16 albardado, 35 acostarse, 850 albugínea, 2574 acromático, 17 aldosterona, 36 acromatina, 18 almohadilla, 38 acromion, 19 almohadilla carpiana, 39 acrosoma, 20 almohadilla córnea, 40 ACTH, 1335 almohadilla dental, 41 actina, 21 almohadilla dentaria, 41 actina F, 22 almohadilla digital, 42 actina G, 23 almohadilla metacarpiana, 43 actitud, 24 almohadilla metatarsiana, 44 acueducto cerebral, 25 almohadilla tarsiana, 45 acueducto de Silvio, 25 alocórtex, 46 acueducto mesencefálico, 25 alto de cola, 2260 adamantoblasto, 59 altura a la punta de la espalda, 56 adenohipófisis, 26 altura anterior de la espalda, 56 ADH, 1336 altura del esternón, 47 adipocito, 27 altura del pecho, 48 ADN, 12 altura del tórax, 48 ADN nucleosómico, 28 alunarado, 49 ADNn, 28 -

LESSON 2 DEVELOPMENT of the PHARYNGEAL ARCHES & POUCHES Objectives by the End of This Lesson You Should Be Able To: 1
LESSON 2 DEVELOPMENT OF THE PHARYNGEAL ARCHES & POUCHES Objectives By the end of this lesson you should be able to: 1. Describe the development of pharyngeal arches 2. Describe the development of pharyngeal pouches 3. Describe the derivatives of pharyngeal arches and pouches 4. Describe the development of the tongue 5. Describe the development of the face 6. Describe the development of the thyroid gland 7. Describe the development of the nasal cavity DEVELOPMENT OF THE PHARYNGEAL APPARATUS In the 4th and 5th week of the development, the formation of the pharyngeal (branchial) arches in the head and neck region contributes greatly to the external appearance of the embryo. • The pharyngeal arches form as the masses of mesenchymal tissue which are invaded by the cranial neural crest cells. • Each pharyngeal arch is externally covered by the ectoderm and internally by the endoderm . • The pharyngeal arches are separated by deep ectodermal clefts called pharyngeal clefts (grooves) . • The endoderm of the pharynx, which lines the internal surface of pharyngeal arches, passes into evaginations called the pharyngeal pouches . Pharyngeal arches – 5th week 1. Pharyngeal arches 2. Lens placode 3. Pericardial swelling 4. Pharyngeal clefts 5. Hand bud Derivatives of pharyngeal pouches 1. External auditory meatus 2. Auditory tube 3. Primary tympanic cavity 4. Cervical sinus 5. Inferior parathyroid gland 6. Thymus 7. Palatine tonsil 8. Superior parathyroid gland 9. Ultimobranchial body pouches 1. Auditory tube 2. Foramen cecum 3. Palatine tonsil 4. Ventral side of pharynx 5. Tympanic cavity 6. Thyroid gland 7. Ultimobranchial body 8. Foregut 9. Thymus 10. Inferior parathyroid gland 11. -

Surgical Anatomy of the Paranasal Sinus M
13674_C01.qxd 7/28/04 2:14 PM Page 1 1 Surgical Anatomy of the Paranasal Sinus M. PAIS CLEMENTE The paranasal sinus region is one of the most complex This chapter is divided into three sections: develop- areas of the human body and is consequently very diffi- mental anatomy, macroscopic anatomy, and endoscopic cult to study. The surgical anatomy of the nose and anatomy. A basic understanding of the embryogenesis of paranasal sinuses is published with great detail in most the nose and the paranasal sinuses facilitates compre- standard textbooks, but it is the purpose of this chapter hension of the complex and variable adult anatomy. In to describe those structures in a very clear and systematic addition, this comprehension is quite useful for an accu- presentation focused for the endoscopic sinus surgeon. rate evaluation of the various potential pathologies and A thorough knowledge of all anatomical structures their managements. Macroscopic description of the and variations combined with cadaveric dissections using nose and paranasal sinuses is presented through a dis- paranasal blocks is of utmost importance to perform cussion of the important structures of this complicated proper sinus surgery and to avoid complications. The region. A correlation with intricate endoscopic topo- complications seen with this surgery are commonly due graphical anatomy is discussed for a clear understanding to nonfamiliarity with the anatomical landmarks of the of the nasal cavity and its relationship to adjoining si- paranasal sinus during surgical dissection, which is con- nuses and danger areas. A three-dimensional anatomy is sequently performed beyond the safe limits of the sinus. -
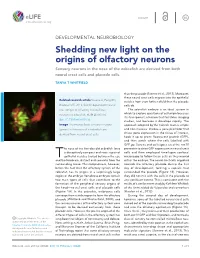
Shedding New Light on the Origins of Olfactory Neurons Sensory Neurons in the Nose of the Zebrafish Are Derived from Both Neural Crest Cells and Placode Cells
INSIGHT elife.elifesciences.org DEVELOPMENTAL NEUROBIOLOGY Shedding new light on the origins of olfactory neurons Sensory neurons in the nose of the zebrafish are derived from both neural crest cells and placode cells. TANYA T WHITFIELD than the placode (Saxena et al., 2013). Moreover, these neural crest cells migrate into the epithelial Related research article Saxena A, Peng BN, vesicles from even further afield than the placode Bronner ME. 2013. Sox10-dependent neural cells do. crest origin of olfactory microvillous The zebrafish embryo is an ideal system in neurons in zebrafish. eLife 2:e00336. which to explore questions of cell origin because it is transparent, a feature that facilitates imaging doi: 10.7554/elife.00336 studies, and because it develops rapidly. The Image The microvillous sensory neurons approach adopted by the Caltech team is simple (green) in the nose of a zebrafish are and non-invasive: choose a gene promoter that derived from neural crest cells drives gene expression in the tissue of interest, hook it up to green fluorescent protein (GFP), and then watch where the cells labelled with GFP go. Saxena and colleagues used the sox10 he nose of the five-day old zebrafish larva promoter to drive GFP expression in neural crest is deceptively compact and neat: a pair of cells and then employed time-lapse confocal Tepithelial vesicles tucked between the eye microscopy to follow these cells as they moved and the forebrain, distinct and separate from the within the embryo. The neural crest cells migrated surrounding tissue. This compactness, however, towards the olfactory placode during the first belies the fact that the olfactory system of the day of development, forming a capsule that zebrafish has its origins in a surprisingly large surrounded the placode (Figure 1B).