AP-2-Null Cells Disrupt Morphogenesis of the Eye, Face, and Limbs in Chimeric Mice
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Syndromic and Complex Craniosynostosis: Craniosynostosis: and Complex Syndromic
Syndromic and Complex Craniosynostosis: Craniosynostosis: and Complex Syndromic Uitnodiging Voor het bijwonen van de openbare verdediging van het proefschrift Syndromic and Complex Craniosynostosis: Skull and brain abnormalities Syndromic and Complex Craniosynostosis: in relation to intracranial hypertension Skull and brain abnormalities in relation to intracranial hypertension door Bianca F.M. Rijken Bianca F. M. Rijken Skull and brain abnormalities in relation to intracranial hypertension intracranial to abnormalitiesSkull in relation and brain op vrijdag 8 april 2016 om 13:30 uur Senaatszaal, Erasmus Building Erasmus Universiteit Rotterdam Burgemeester Oudlaan 50 3062 PA Rotterdam Na afloop van de promotie bent u van harte uitgenodigd voor de receptie Bianca F.M. Rijken De Ruyterstraat 10b 3071 PJ Rotterdam [email protected] 06-33148619 Paranimfen Sun-Jine van Bezooijen [email protected] 06-33048922 Hoa Ha [email protected] 06-14911149 Bianca F. M. Rijken Bianca F. Syndromic and Complex Craniosynostosis: Skull and brain abnormalities in relation to intracranial hypertension Bianca F. M. Rijken ISBN 978-94-6299-319-8 Layout & printing Ridderprint BV - www.ridderprint.nl Cover design Ridderprint BV - www.ridderprint.nl Copyright 2016 Bianca Francisca Maria Rijken All rights reserved. No part of this book may be reproduced, stored in a retrieval system or transmitted in any form or by any means, without prior permission of the author. Sponsors: Syndromic and Complex Craniosynostosis: Skull and brain abnormalities in relation to intracranial hypertension Syndromale en complexe craniosynostose: Schedel en brein afwijkingen in relatie tot intracraniële hypertensie Proefschrift ter verkrijging van de graad van doctor aan de Erasmus Universiteit Rotterdam op gezag van de rector magnificus Prof.dr. -

Letter to Editor Sep 2012; Vol 22 (No 3), Pp: 432-433
Iran J Pediatr Letter to Editor Sep 2012; Vol 22 (No 3), Pp: 432-433 pregnancy. First born baby died at the age of 10 Isolated Lower Limb Phocomelia – a Rare postnatal day, cause is unknown, but he was Limb Malformation apparently not having any congenital malformation. Physical examination revealed weight 4.8 kg, length 54.5 cm, head Priyanka Bansal*, MD; Akhil Bansal, MD, and Shitalmala Devi, MD circumference 36.5 cm, no facial dysmorphism, spine normal. Only deformity was phocomelia of Jawaharlal Nehru Medical College, Department of Pathology, left lower limb (Fig. 1). Systemic examination India was normal. X ray pelvis with both lower limbs Received: Jan 29, 2011; Accepted: Mar 24, 2012 showed left lower limb showed absent femur, tibia and fibula, a single tarsal bone visualized, Phocomelia, ie the absence or severe hypoplasia distal foot appears grossly normal. Right hip and of the long tubular bones with more or less femur do not reveal any abnormality. Chest X ray intact hands and or feet, is widely known to be was absolutely normal and abdominal the most spectacular finding of thalidomide sonography abdomen and cranianl showed no embryopathy[1]. It may be complete in the form abnormality. 2-dimensional echocardiography that proximal and distal bones of limb are absent was done to rule out congenital heart defect, or may be incomplete when either proximal or which was also normal. distal bones are missing. It is known to occur in Phocomelia[2] in the complete form, the arm some familial syndromes such as Roberts and forearm are absent in the upper limb and syndrome[10], the DK Phocomelia syndrome[8] the thigh and leg are absent in the lower limb and in a few other extremely rare syndromes. -
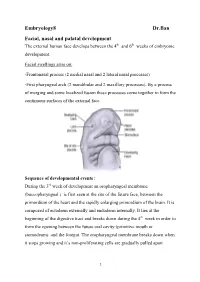
Embryology8 Dr.Ban Facial, Nasal and Palatal Development the External Human Face Develops Between the 4Th and 6Th Weeks of Embryonic Development
Embryology8 Dr.Ban Facial, nasal and palatal development The external human face develops between the 4th and 6th weeks of embryonic development. Facial swellings arise on: -Frontonasal process (2 medial nasal and 2 lateral nasal processes) -First pharyngeal arch (2 mandibular and 2 maxillary processes). By a process of merging and some localized fusion these processes come together to form the continuous surfaces of the external face. Sequence of developmental events : During the 3rd week of development an oropharyngeal membrane (buccopharyngeal ) is first seen at the site of the future face, between the primordium of the heart and the rapidly enlarging primordium of the brain. It is composed of ectoderm externally and endoderm internally. It lies at the beginning of the digestive tract and breaks down during the 4th week in order to form the opening between the future oral cavity (primitive mouth or stomodeum) and the foregut. The oropharyngeal membrane breaks down when it stops growing and it’s non-proliferating cells are gradually pulled apart 1 because they cannot fill the expanding area.The tissues around it expand very rapidly. The face develops from five primordia that appear in the fourth week: the frontonasal prominence, the two maxillary swellings, and the two mandibular swellings. The external face forms from two sources that surround the oropharyngeal membrane 1-Tissues of the frontonasal process that cover the forebrain, predominantly of neural crest origin. 2-The tissues of the first (or mandibular) pharyngeal arch, of mixed mesoderm and neural crest origin Face initially formed by 5 mesenchymal swellings ( prominences): Two mandibular prominences Two maxillary prominences Frontonasal prominence (midline structure is a single structure that is ventral to the forebrain. -

Brachycephaly and Syndactyly: Apert's Syndrome
Letters to Editor keratinocyte differentiation, it is possible that acitretin may Acne is the most common cutaneous association of Apert’s inhibit viral replication and assembly within the affected syndrome. cells.[3] Oral retinoids have been used with remarkable improvement in the management of extensive and An eight-month-old boy born of nonconsanguineous recalcitrant warts without recurrences.[3,4] parents presented with multiple congenital anomalies. The mother’s antenatal period was uneventful, with no history Epidermodysplasia verruciformis, an inherited disorder of any infectious illness, medication(s) exposure or addiction characterized by widespread and persistent infection with during that period. Family history was non-contributory. HPV, pityriasis versicolor-like lesions and reddish plaques, The patient’s anomalies included craniofacial abnormality has shown dramatic improvement with oral acitretin when and syndactyly of all four limbs. The child had a typical used alone or in combination.[5] Our patient tolerated the facial appearance with a short, wide head (brachycephaly), oral acitretin well with gratifying clinical results without any frontal bossing, retruded (sunken) mid-face, depressed nasal major side effects or recurrences. Hence, oral acitretin can bridge, upward slanting of the eyes, low-set ears and a low be considered as a useful treatment option for extensive posterior hairline [Figure 1]. Examination of the upper and recalcitrant warts. lower limbs showed symmetrical syndactyly leading to the fusion of all the fingers and toes [Figure 2]. The child had DD.. SS.. KKruparupa SShankar,hankar, RRachanaachana SShilpakarhilpakar both growth and mental retardation. Department of Dermatology, Manipal Hospital, Bangalore, India Apert’s syndrome is specifically related to the paternal AAddressddress fforor ccorrespondence:orrespondence: Dr. -

Medically Necessary Orthodontic Treatment – Dental
UnitedHealthcare® Dental Coverage Guideline Medically Necessary Orthodontic Treatment Guideline Number: DCG003.08 Effective Date: November 1, 2020 Instructions for Use Table of Contents Page Related Medical Policy Coverage Rationale ....................................................................... 1 • Orthognathic (Jaw) Surgery Definitions ...................................................................................... 1 Applicable Codes .......................................................................... 3 Description of Services ................................................................. 3 References ..................................................................................... 3 Guideline History/Revision Information ....................................... 4 Instructions for Use ....................................................................... 4 Coverage Rationale Orthodontic treatment is medically necessary when the following criteria have been met: All services must be approved by the plan; and The member is under the age 19 (through age 18, unless the member specific benefit plan document indicates a different age); and Services are related to the treatment of a severe craniofacial deformity that results in a physically Handicapping Malocclusion, including but not limited to the following conditions: o Cleft Lip and/or Cleft Palate; o Crouzon Syndrome/Craniofacial Dysostosis; o Hemifacial Hypertrophy/Congenital Hemifacial Hyperplasia; o Parry-Romberg Syndrome/Progressive Hemifacial Atrophy; -

Radial, Renal and Craniofacial Anomalies: Baller-Gerold Syndrome
Published online: 2020-01-15 Free full text on www.ijps.org Case Report Radial, renal and craniofacial anomalies: Baller-Gerold syndrome Jyotsna Murthy, Ramesh Babu1, Padmasani Venkat Ramanan2 Department of Plastic Surgery, Director of Cleft and Craniofacial Center, 1Consultant Pediatric Urologist, 2Department of Pediatrics, Sri Ramachandra Medical College and Research Institute, Porur, Chennai, Tamil Nadu, India Address for correspondence: Dr. Jyotsna Murthy, Department of Plastic Surgery, Sri Ramachandra Medical College and Research Institute, Porur, Chennai - 600 116, Tamil Nadu, India. E-mail: [email protected] ABSTRACT The Baller-Gerold syndrome is a rare syndrome with very few cases published in literature. Craniosynostosis and radial aplasia are striking features, easy to diagnose. However, there are many differential diagnoses. Often, the question raised is whether the Baller-Gerald syndrome is a distinct entity. We report a patient with Þ ndings of craniosynostosis and radial aplasia consistent with the diagnosis of the Baller-Gerold syndrome. Genotypic heterogeneity could possibly underlie the phenotypic variability exhibited by these cases. KEY WORDS Baller-Gerold syndrome, craniosynostosis, crossed ectopia, ectopic kidneys, microcephaly, radial agenesis, radial club hand, reß ux, renal agenesis, vesico ureteric reß ux INTRODUCTION anal (imperforated anus, anterior anus, perineal fistulae), nervous system (polymicrogria, hydrocephalus, agenesis aller[1] described a female with oxycephaly and of corpus callosum or seizure disorder) and skeletal absent radius whose parents were third cousins. (joint limitation, rib fusion, flat vertebrae and absent BGerold[2] described a brother and sister, aged middle phalanx). They are divided into two groups - those 16 years and two days, respectively with tower skull, with craniosynostosis and radial defect alone and those radial aplasia and small ulna. -

Prenatal Ultrasonography of Craniofacial Abnormalities
Prenatal ultrasonography of craniofacial abnormalities Annisa Shui Lam Mak, Kwok Yin Leung Department of Obstetrics and Gynaecology, Queen Elizabeth Hospital, Hong Kong SAR, China REVIEW ARTICLE https://doi.org/10.14366/usg.18031 pISSN: 2288-5919 • eISSN: 2288-5943 Ultrasonography 2019;38:13-24 Craniofacial abnormalities are common. It is important to examine the fetal face and skull during prenatal ultrasound examinations because abnormalities of these structures may indicate the presence of other, more subtle anomalies, syndromes, chromosomal abnormalities, or even rarer conditions, such as infections or metabolic disorders. The prenatal diagnosis of craniofacial abnormalities remains difficult, especially in the first trimester. A systematic approach to the fetal Received: May 29, 2018 skull and face can increase the detection rate. When an abnormality is found, it is important Revised: June 30, 2018 to perform a detailed scan to determine its severity and search for additional abnormalities. Accepted: July 3, 2018 Correspondence to: The use of 3-/4-dimensional ultrasound may be useful in the assessment of cleft palate and Kwok Yin Leung, MBBS, MD, FRCOG, craniosynostosis. Fetal magnetic resonance imaging can facilitate the evaluation of the palate, Cert HKCOG (MFM), Department of micrognathia, cranial sutures, brain, and other fetal structures. Invasive prenatal diagnostic Obstetrics and Gynaecology, Queen Elizabeth Hospital, Gascoigne Road, techniques are indicated to exclude chromosomal abnormalities. Molecular analysis for some Kowloon, Hong Kong SAR, China syndromes is feasible if the family history is suggestive. Tel. +852-3506 6398 Fax. +852-2384 5834 E-mail: [email protected] Keywords: Craniofacial; Prenatal; Ultrasound; Three-dimensional ultrasonography; Fetal structural abnormalities This is an Open Access article distributed under the Introduction terms of the Creative Commons Attribution Non- Commercial License (http://creativecommons.org/ licenses/by-nc/3.0/) which permits unrestricted non- Craniofacial abnormalities are common. -

Syndromes of the First and Second Branchial Arches, Part 1: Embryology and Characteristic REVIEW ARTICLE Defects
Syndromes of the First and Second Branchial Arches, Part 1: Embryology and Characteristic REVIEW ARTICLE Defects J.M. Johnson SUMMARY: A variety of congenital syndromes affecting the face occur due to defects involving the G. Moonis first and second BAs. Radiographic evaluation of craniofacial deformities is necessary to define aberrant anatomy, plan surgical procedures, and evaluate the effects of craniofacial growth and G.E. Green surgical reconstructions. High-resolution CT has proved vital in determining the nature and extent of R. Carmody these syndromes. The radiologic evaluation of syndromes of the first and second BAs should begin H.N. Burbank first by studying a series of isolated defects: CL with or without CP, micrognathia, and EAC atresia, which compose the major features of these syndromes and allow more specific diagnosis. After discussion of these defects and the associated embryology, we proceed to discuss the VCFS, PRS, ACS, TCS, Stickler syndrome, and HFM. ABBREVIATIONS: ACS ϭ auriculocondylar syndrome; BA ϭ branchial arch; CL ϭ cleft lip; CL/P ϭ cleft lip/palate; CP ϭ cleft palate; EAC ϭ external auditory canal; HFM ϭ hemifacial microsomia; MDCT ϭ multidetector CT; PRS ϭ Pierre Robin sequence; TCS ϭ Treacher Collins syndrome; VCFS ϭ velocardiofacial syndrome adiographic evaluation of craniofacial deformities is nec- major features of the syndromes of the first and second BAs. Ressary to define aberrant anatomy, plan surgical proce- Part 2 of this review discusses the syndromes and their radio- dures, and evaluate the effects of craniofacial growth and sur- graphic features: PRS, HFM, ACS, TCS, Stickler syndrome, gical reconstructions.1 The recent rapid proliferation of and VCFS. -

Lingual Agenesis: a Case Report and Review of Literature
Research Article Clinics in Surgery Published: 09 Mar, 2018 Lingual Agenesis: A Case Report and Review of Literature Mark Enverga, Ibrahim Zakhary* and Abraham Khanafer Department of Oral and Maxillofacial Surgery, University of Detroit Mercy, School of Dentistry, USA Abstract Aglossia is a rare condition characterized by the complete absence of the tongue. Its etiology is still unknown. The underlying pathophysiology involves disruption of the development of the lateral lingual swellings and tuberculum impar during the second month of gestation. In this case report, a 26-year old African American female with aglossia presented to the Detroit Mercy Oral Surgery Clinic. The patient presented for extraction of tooth #28, which was located within a fused bony plate at the floor of the mandible. This study presents a case of aglossia, as well as a critical review of aglossia in current literature. Introduction Aglossia is a rare condition characterized by the complete absence of the tongue. The exact etiology of aglossia is still unknown. Possible etiologic factors during embryogenesis include maternal febrile illness, drug ingestion, hypothyroidism, and cytomegalovirus infection. Heat-induced vascular disruption in the fourth embryonic week and chronic villous sampling performed before 10 weeks of amenorrhea, also called the disruptive vascular hypothesis, may be another possible cause of aglossia [1,2]. The underlying pathophysiology involves disruption of the normal embryogenic development of the lateral lingual swellings and tuberculum impar during the second month of gestation [3]. Most cases of aglossia are associated with other congenital limb malformations and craniofacial abnormalities, such as hypodactyly, adactyly, cleft palate, Pierre Robin sequence, Hanhart syndrome, Moebius syndrome, and facial nerve palsy. -

Illustrated Review of the Embryology and Development of the Facial
REVIEW ARTICLE Illustrated Review of the Embryology and Development of the Facial Region, Part 2: Late Development of the Fetal Face and Changes in the Face from the Newborn to Adulthood P.M. Som and T.P. Naidich ABSTRACT SUMMARY: The later embryogenesis of the fetal face and the alteration in the facial structure from birth to adulthood have been reviewed. Part 3 of the review will address the molecular mechanisms that are responsible for the changes described in parts 1 and 2. art 1 of this 3-part review primarily dealt with the early em- first make contact, each is completely covered by a homoge- Pbryologic development of the face and nasal cavity. Part 2 will neous epithelium. A special epithelium arises at the edge of discuss the later embryonic and fetal development of the face, and each palatal shelf, facilitating the eventual fusion of these changes in facial appearance from neonate to adulthood will be shelves. The epithelium on the nasal cavity surface of the palate reviewed. will differentiate into columnar ciliated epithelium. The epi- thelium on the oral cavity side of the palate will differentiate Formation of the Palate into stratified squamous epithelium. Between the sixth and 12th weeks, the palate is formed from 3 The 2 palatal shelves also fuse with the triangular primary pal- primordia: a midline median palatine process and paired lateral ate anteromedially to form a y-shaped fusion line. The point of palatine processes (Fig 1). In the beginning of the sixth week, fusion of the secondary palatal shelves with the primary palate is merging of the paired medial nasal processes forms the intermax- marked in the adult by the incisive foramen. -

Three-Dimensional Ultrasound Study of Fetal Craniofacial Anatomy
Three-dimensional ultrasound study of fetal craniofacial anatomy N.M. Roelfsema The work presented in this thesis was financially supported by the Netherlands Organization for Scientific Research, grand no: 902-37-116. It was conducted at the department of Obstet- rics and Gynecology and in collaberation with the department of Plastic and Reconstructive Surgery, Erasmus MC, Rotterdam, The Netherlands J.E. Jurriaanse Stichting and GE Healthcare financially supported the printing of this thesis The following parts of this publication have been published previously and have been repro- duced with permission from the publishers: Elsevier Ltd.(Roelfsema et al. Three-dimensional sonographic measurement of normal fetal brain volume during the second half of pregnancy. Am J Obstet Gynecol 2004;190:275-80) and the International Society of Ultrasound in Obstet- rics and Gynecology (Roelfsema et al. Three-dimensional ultrasonography of prenatal skull base development. Ultrasound Obstet Gynecol 2007:29:372-7; Roelfsema et al. Craniofacial variability index determined by three-dimensional ultrasound in isolated versus syndromal cleft lip/palate. Ultrasound Obstet Gynecol 2007;29:265-70; Roelfsema et al. Craniofacial variability index in utero; a three-dimensional ultrasound study. Ultrasound Obstet Gynecol 2007;29:258-64; Roelfsema et al. Three-dimensional sonographic determination of normal fetal mandibular and maxillary development during the second half of pregnancy. Ultrasound Obstet Gynecol 2006;28:950-7; Dikkeboom et al. The role of three-dimensional ultrasound in visualizing the fetal cranial sutures and fontanels during the second half of pregnancy. Ultra- sound Obstet Gynecol 2004;24:412-6). Front cover: © N.M. Roelfsema © 2007, N.M. -

Attenuation of Signaling Pathways Stimulated by Pathologically Activated FGF-Receptor 2 Mutants Prevents Craniosynostosis
Attenuation of signaling pathways stimulated by pathologically activated FGF-receptor 2 mutants prevents craniosynostosis V. P. Eswarakumar*, F. Ozcan*¨ †, E. D. Lew*, J. H. Bae*, F. Tome´ *, C. J. Booth‡, D. J. Adams§, I. Lax*, and J. Schlessinger*¶ *Department of Pharmacology and ‡Section of Comparative Medicine, Yale University School of Medicine, New Haven, CT 06520; and §Department of Orthopaedic Surgery, University of Connecticut Health Center, Farmington, CT 06030 Contributed by J. Schlessinger, October 19, 2006 (sent for review September 22, 2006) Craniosynostosis, the fusion of one or more of the sutures of the heparin/FGFR complex results in FGFR dimerization and ac- skull vault before the brain completes its growth, is a common (1 tivation (11–14). The Crouzon Cys342Tyr mutation disrupts the in 2,500 births) craniofacial abnormality, Ϸ20% of which occur- structure of D3, abrogating the ligand-binding capacity of the rences are caused by gain-of-function mutations in FGF receptors extracellular domain of FGFR2c. In addition, Cys-278, which (FGFRs). We describe a genetic and pharmacological approach for normally forms an intramolecular disulfide bond with Cys-342, the treatment of a murine model system of Crouzon-like cranio- becomes unpaired. The unpaired Cys-278 instead forms a disul- synostosis induced by a dominant mutation in Fgfr2c. Using ge- fide bond with Cys-278 of a neighboring similarly mutated netically modified mice, we demonstrate that premature fusion of FGFR2c molecule intermolecularly resulting in the formation of sutures mediated by Crouzon-like activated Fgfr2c mutant is pre- constitutively activated stable homodimers that are unable to vented by attenuation of signaling pathways by selective uncou- bind FGF (9, 10, 15).