OKELLO-THESIS.Pdf
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Te2, Part Iii
TERMINOLOGIA EMBRYOLOGICA Second Edition International Embryological Terminology FIPAT The Federative International Programme for Anatomical Terminology A programme of the International Federation of Associations of Anatomists (IFAA) TE2, PART III Contents Caput V: Organogenesis Chapter 5: Organogenesis (continued) Systema respiratorium Respiratory system Systema urinarium Urinary system Systemata genitalia Genital systems Coeloma Coelom Glandulae endocrinae Endocrine glands Systema cardiovasculare Cardiovascular system Systema lymphoideum Lymphoid system Bibliographic Reference Citation: FIPAT. Terminologia Embryologica. 2nd ed. FIPAT.library.dal.ca. Federative International Programme for Anatomical Terminology, February 2017 Published pending approval by the General Assembly at the next Congress of IFAA (2019) Creative Commons License: The publication of Terminologia Embryologica is under a Creative Commons Attribution-NoDerivatives 4.0 International (CC BY-ND 4.0) license The individual terms in this terminology are within the public domain. Statements about terms being part of this international standard terminology should use the above bibliographic reference to cite this terminology. The unaltered PDF files of this terminology may be freely copied and distributed by users. IFAA member societies are authorized to publish translations of this terminology. Authors of other works that might be considered derivative should write to the Chair of FIPAT for permission to publish a derivative work. Caput V: ORGANOGENESIS Chapter 5: ORGANOGENESIS -

Nhbs Monthly Catalogue New and Forthcoming Titles Issue: 2012/05 May 2012 [email protected] +44 (0)1803 865913
nhbs monthly catalogue new and forthcoming titles Issue: 2012/05 May 2012 www.nhbs.com [email protected] +44 (0)1803 865913 Welcome to the May 2012 edition of the NHBS Monthly Catalogue. This monthly Zoology: update contains all of the wildlife, science and environment titles added to nhbs.com in Mammals the last month. Birds Editor's Picks - New in Stock this Month Reptiles & Amphibians Fishes ● The Art of Tracking Animals Invertebrates ● Australian Carnivorous Plants Palaeontology ● Bat Surveys: Good Practice Guidelines General Natural History ● The Behavior Guide to Africal Mammals Regional & Travel ● Biodiversity in Dead Wood Botany & Plant Science ● Conifer Moths of the British Isles: A Field Guide to Coniferous-feeding Lepidoptera Animal & General Biology ● Dolphin Confidential: Confessions of a Field Biologist Evolutionary Biology ● Evolutionary History of Bats: Fossils, Molecules and Morphology Ecology ● A Field Guide to Reptiles and Amphibians of Israel Habitats & Ecosystems ● Games Primates Play: An Undercover Investigation of the Evolution and Conservation & Biodiversity Economics of Human Relationships Environmental Science ● The Great Animal Orchestra: Finding the Origins of Music in the World's Wild Places Physical Sciences ● How to Be a Better Birder Sustainable Development ● Penguin-Pedia: Photographs and Facts from One Man's Search for the Penguins Data Analysis of the World Reference ● Polar Bears: A Complete Guide to Their Biology and Behaviour ● The Wildlife Techniques Manual (2-Volume Set) ● A World of Insects: The Harvard University Press Reader Find out more about services for libraries and organisations: NHBS LibraryPro Best wishes, -The NHBS Team View this Monthly Catalogue as a web page or save/print it as a .pdf document. -

Nessa Carey the Challenges in Turning Epigenetic Hope Into
Nessa Carey The Challenges in Turning Epigenetic Hope into Clinical Reality Confidential and Internal to Pfizer - subject to works council and/or union consultations and other legal requirements. 0 All Hail The Double Helix “Today we are learning the language in which God created life” “The outstanding achievement .. in terms of human history” Confidential and Internal to Pfizer - subject to works council and/or union consultations and other legal requirements. But There Must Be More Confidential and Internal to Pfizer - subject to works council and/or union consultations and other legal requirements. How Do We Know? EYE OF SCIENCE / SPL / BARCROFT MEDIA Where genetic identity ≠ phenotypic identity….. EPIGENETICS Confidential and Internal to Pfizer - subject to works council and/or union consultations and other legal requirements. DNA Is Not A Lonely Molecule Lodish et al., 2000 Confidential and Internal to Pfizer - subject to works council and/or union consultations and other legal requirements. Confidential and Internal to Pfizer - subject to works council and/or union consultations and other legal requirements. Confidential and Internal to Pfizer - subject to works council and/or union consultations and other legal requirements. Confidential and Internal to Pfizer - subject to works council and/or union consultations and other legal requirements. Confidential and Internal to Pfizer - subject to works council and/or union consultations and other legal requirements. Confidential and Internal to Pfizer - subject to works council and/or union consultations and other legal requirements. Confidential and Internal to Pfizer - subject to works council and/or union consultations and other legal requirements. Confidential and Internal to Pfizer - subject to works council and/or union consultations and other legal requirements. -

Syndromes of the First and Second Branchial Arches, Part 1: Embryology and Characteristic REVIEW ARTICLE Defects
Syndromes of the First and Second Branchial Arches, Part 1: Embryology and Characteristic REVIEW ARTICLE Defects J.M. Johnson SUMMARY: A variety of congenital syndromes affecting the face occur due to defects involving the G. Moonis first and second BAs. Radiographic evaluation of craniofacial deformities is necessary to define aberrant anatomy, plan surgical procedures, and evaluate the effects of craniofacial growth and G.E. Green surgical reconstructions. High-resolution CT has proved vital in determining the nature and extent of R. Carmody these syndromes. The radiologic evaluation of syndromes of the first and second BAs should begin H.N. Burbank first by studying a series of isolated defects: CL with or without CP, micrognathia, and EAC atresia, which compose the major features of these syndromes and allow more specific diagnosis. After discussion of these defects and the associated embryology, we proceed to discuss the VCFS, PRS, ACS, TCS, Stickler syndrome, and HFM. ABBREVIATIONS: ACS ϭ auriculocondylar syndrome; BA ϭ branchial arch; CL ϭ cleft lip; CL/P ϭ cleft lip/palate; CP ϭ cleft palate; EAC ϭ external auditory canal; HFM ϭ hemifacial microsomia; MDCT ϭ multidetector CT; PRS ϭ Pierre Robin sequence; TCS ϭ Treacher Collins syndrome; VCFS ϭ velocardiofacial syndrome adiographic evaluation of craniofacial deformities is nec- major features of the syndromes of the first and second BAs. Ressary to define aberrant anatomy, plan surgical proce- Part 2 of this review discusses the syndromes and their radio- dures, and evaluate the effects of craniofacial growth and sur- graphic features: PRS, HFM, ACS, TCS, Stickler syndrome, gical reconstructions.1 The recent rapid proliferation of and VCFS. -

Trávicí Systém
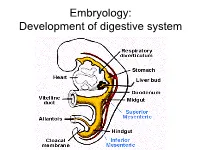
Embryology: Development of digestive system Embryo folding – incorporation of endoderm to form primitive gut. Outside of embryo – yolk sac and allantois. Vitelline duct Stomodeum (primitive mouth) the oral cavity + the salivary glands Proctodeum primitive anal pit Primitive gut whole digestive tube + accessory glands pharynx forgut midgut hindgut • The epithelium and glandular cells of associated glands of the gastrointestinal tract develop from endoderm • The connective tissue, muscle tissue and mesothelium are derived from splanchnic mesoderm • The enteric nervous system develops from neural crest primitive gut foregut midgut hindgut pharyngeal above ductus cloacal membrane omphalomesentericus membrane and yolk sack Derivatives of forgut – pharynx, esophagus (+ respiratory diverticul), stomach, cranial part of duodenum midgut – caudal part of duodenum (+ liver, gall bladder, pancreas), small intestine and part of large intestine (to the flexura coli sin.) hindgut – large intestine (from flexura coli sin.), rectum, upper part of anal canal Oral cavity • primitive mouth pit – stomodeum • lined with ectoderm • surrounded by: - processus frontalis (single) - proc. maxillares (paired) - proc. mandibulares (paired) • pharyngeal membrane (it ruptures during the 4th week, primitive gut communicates with amnionic cavity Pharyngeal (branchial) apparatus Pharyngeal arches • appear in weeks 4 - 5 • on the ventral side of the pharyngeal gut. • each arch has cartilage, cranial nerve, aortic arch artery and muscle • pharyngeal clefts and pouches -

Anything but Junk Delving Deep Into the Dark Matter of the Human Genome
David Martindill Anything but junk Delving deep into the dark matter of the human genome Most biology students know who discovered the This is much larger than that of many other species, Key words structure of DNA. But few can recall Fred Sanger’s for example the nematode worm, C. elegans, which has a genome around 3% the size of ours. gene contributions to genetics – and what about those genome of Francis Collins and John Sulston? DNA It is now more than a decade since the Human medicine Genome Project concluded. Employing the technique of DNA sequencing developed by Sanger, an international consortium of scientists, including Collins and Sulston, determined the entire sequence of our genome, a code of 3.3 billion letters. Wellcome Trust Wellcome A tiny section of the human genome sequence. A staggering 3.3 billion letters long, it would take over 90 years, day and night, to read the complete sequence at a rate of one letter per second! Genome Research Ltd. A comparison of genome size and gene number in DNA sequencing machines at the Wellcome Trust Sanger Institute humans and the nematode worm, C. elegans 6 Catalyst April 2015 www.catalyststudent.org.uk The alphabet of molecular biology DNA has a simplified alphabet of four letters, A, T, C and G. These are known as ‘bases’ and are read by the cell in groups of three. Each triplet encodes a specific amino acid, of which there are twenty different types, which the cell then joins together to form a protein. The function of a protein, encoded by a specific stretch of DNA called a gene, is entirely dependent on the sequence of its amino acids. -
Cleft Palate Are Common Defects That Result in Abnormal Facial Appearance and Defective Speech
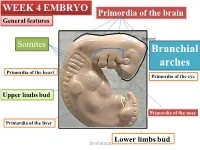
WEEK 4 EMBRYO Primordia of the brain General features shatarat Somites Branchial arches Primordia of the heart Primordia of the eye Upper limbs bud Primordia of the nose Primordia of the liver Lower limbs bud Dr.shatarat The most important feature in the development of the head and neck is the Formation of THE PHARYNGEAL OR BRANCHIALARCHES shatarat Dr.shatarat Is it branchial or is it pharyngeal arch? development of pharyngeal arches resembles formation of gills in fish However, in the human embryo real gills (branchia) are never formed. Therefore, the term pharyngeal arches has been adopted for the human embryo Dr.shatarat shatarat THE PHARYNGEALARCHES appear in the fourth and fifth weeks of development Dr.shatarat Why the appear? Migration of cells from epiblast Dr.shatarat 1-PARAXIALMESODERM 2-LATERALPLATE MESODERM 3-NEURALCREST Dr.shatarat Migration of the cells from the occipital Myotomes into the future mouth to form the tongue Occipital somites This is an explanation to how the arches appear…. as a result of migration of the cells from the medial mesoderm (somites) into tongue the regions of the future head and neck. As we mentioned there are other reasons Dr.shatarat In a cross section of the embryo in the area of the head and neck The following can be noticed THE PHARYNGEALARCHES shatarat THE PHARYNGEALARCHES are separated by deep clefts known as PHARYNGEAL CLEFTS with development of the arches and clefts, a number of outpocketings, The pharyngeal pouches appear Dr.shatarat 1-PHARYNGEAL ARCHs Dr.shatarat 6 However, The fifth and sixth arches are rudimentary and are not visible on the surface of the embryo T h e y a re n u m b e r e d i n c r a n i o ca ud a During the fifth week, the second pharyngeal l s e arch enlarges and overgrows the third and qu fourth arches, forming the ectodermal e n c depression called cervical sinus e Dr.shatarat Each pharyngeal arch consists of: 1-surface ECTODERM 2-a core of MESENCHYMALtissue 3- epithelium of ENDODERMAL origin Each pharyngeal arch contains: 1- An artery that arises from the primordial heart A. -
Ectodermal Pharyngeal Clefts
Embryology: Development of digestive system Flecture of embryo – incorporation of endoderm to form primitive gut. Outside of embryo – yolk sac and allantois. Vitelline duct Stomodeum (primitive mouth) the oral cavity + the salivary glands Proctodeum primitive anal pit Primitive gut whole digestive tube + accessory glands orofacial membrane Tracheo- esophageal septum Proctodeum cloacal membrane Primitive gut pharynx forgut midgut hindgut Derivatives of foregut – pharynx, (+ respiratory diverticle),esophagus, stomach, cranial part of duodenum, (+ liver, gall bladder pancreas) midgut – caudal part of duodenum, small intestine and part of large intestine (1/3 of colon transv.) hindgut – the rest of large intestine, rectum, upper part of the anal canal Tissues in GIT • The epithelium of gut and glandular cells of associated glands of the gastrointestinal tract develop from endoderm • The connective tissue, muscle tissue and mesothelium derive from splanchnic mesoderm • The enteric nervous system develops from neural crest primitive gut foregut midgut hindgut from above ductus to cloacal pharyngeal omphalomesentericus membrane membrane and yolk sack Pharyngeal membrane liver, bile duct, pancreas Rectum Origin: forgut, midgut. hindgut Oral cavity • primitive mouth pit – stomodeum • lined with ectoderm • surrounded by: - processus frontalis (single) - proc. maxillares (paired) - proc. mandibulares (paired) • orophacial membrane (it ruptures during the 4th week, primitive gut communicates with amnionic cavity) Pharyngeal (branchial) apparatus -

Facial and Palatal Development
11. FACIAL AND PALATAL DEVELOPMENT Letty Moss-Salentijn DDS, PhD Dr. Edwin S.Robinson Professor of Dentistry (in Anatomy and Cell Biology) E-mail: [email protected] READING ASSIGNMENT: Larsen 3rd edition: p.352; pp.365-371; 398-404. SUMMARY: The external human face develops between the 4th and 6th weeks of embryonic development. Facial swellings arise on the frontonasal process (2 medial nasal and 2 lateral nasal processes) and the first pharyngeal arch (2 mandibular and 2 maxillary processes). By a process of merging and some localized fusion these processes come together to form the continuous surfaces of the external face. The primary palate is formed in this period by fusion/merging of the medial nasal and maxillary processes. Subsequently, between 6th and 12th embryonic/fetal weeks, the secondary palate is formed as the result of fusion between palatal processes growing from the oral surfaces of the maxillary processes. Each merging and fusion site is also the site of a potential facial or palatal cleft. LEARNING OBJECTIVES You should be able to: a. Demonstrate on a frontal image of a human face those parts of the face that are formed by contributions from the frontonasal process and those that are formed by contributions from the first pharyngeal arch. b. Describe the following as to site, composition, time of appearance, and fate: oropharyngeal membrane, oronasal membrane. c. List the derivatives of the four pairs of facial processes and the pair of palatal processes. d. Describe the development of the nose and primary palate. e. Explain the differences between the processes of merging and fusion. -

High-Yield Embryology 4
LWBK356-FM_pi-xii.qxd 7/14/09 2:03 AM Page i Aptara Inc High-Yield TM Embryology FOURTH EDITION LWBK356-FM_pi-xii.qxd 7/14/09 2:03 AM Page ii Aptara Inc LWBK356-FM_pi-xii.qxd 7/14/09 2:03 AM Page iii Aptara Inc High-Yield TM Embryology FOURTH EDITION Ronald W. Dudek, PhD Professor Brody School of Medicine East Carolina University Department of Anatomy and Cell Biology Greenville, North Carolina LWBK356-FM_pi-xii.qxd 7/14/09 2:03 AM Page iv Aptara Inc Acquisitions Editor: Crystal Taylor Product Manager: Sirkka E. Howes Marketing Manager: Jennifer Kuklinski Vendor Manager: Bridgett Dougherty Manufacturing Manager: Margie Orzech Design Coordinator: Terry Mallon Compositor: Aptara, Inc. Copyright © 2010, 2007, 2001, 1996 Lippincott Williams & Wilkins, a Wolters Kluwer business. 351 West Camden Street 530 Walnut Street Baltimore, MD 21201 Philadelphia, PA 19106 Printed in China All rights reserved. This book is protected by copyright. No part of this book may be reproduced or transmitted in any form or by any means, including as photocopies or scanned-in or other electronic copies, or utilized by any information storage and retrieval system without written permission from the copyright owner, except for brief quotations embodied in critical articles and reviews. Materials appear- ing in this book prepared by individuals as part of their official duties as U.S. government employees are not covered by the above-mentioned copyright. To request permission, please contact Lippincott Williams & Wilkins at 530 Walnut Street, Philadelphia, PA 19106, via email at [email protected], or via website at lww.com (products and services). -

We Are the 98% the Human Genome Than Meets the Eye JOHN PARRINGTON Nathaniel Comfort Unpicks the Metaphors in a Trio of Oxford Univ
SPRING BOOKS COMMENT systems evolve and can be locked into tra- huge value remains in his wisdom and his and economics. Stern is a big thinker, used jectories by incumbent industries, unless experience of international debates. to the broad sweeps of economic develop- strong pressures force a change in course. However, Stern’s coverage of lessons from ment and global issues. But the crux is local These strands of economics are crucial to economic history and theory is messier. It people and businesses. Voters might like the understanding the energy–climate nexus meanders through market failures, policy idea of clean energy, but oppose wind farms and policies for transformation. The econom- uncertainties, the European Union’s emis- next door; back emission reductions and pro- ics mainstream is still largely in thrall to the sions-trading system, the debate over reserves fess support for market-based solutions, but idea that competitive markets drive innova- of fossil fuels that must be left unburnt to sat- oppose increased energy prices. There is little tion, but liberalization of the energy sector isfy emissions targets, shale gas, the German on energy prices in Why Are We Waiting?, but destroyed UK energy research and develop- nuclear phase-out, failed Chinese dams and climate policy in Brussels and Washington ment. The energy literature explains why, but more. There are trenchant points about six DC is concerned with little else. Stern hardly touches on this and thus misses market failures and the six areas of policy The real challenge in controlling climate a chance to help to educate fellow economists. -

26 April 2010 TE Prepublication Page 1 Nomina Generalia General Terms
26 April 2010 TE PrePublication Page 1 Nomina generalia General terms E1.0.0.0.0.0.1 Modus reproductionis Reproductive mode E1.0.0.0.0.0.2 Reproductio sexualis Sexual reproduction E1.0.0.0.0.0.3 Viviparitas Viviparity E1.0.0.0.0.0.4 Heterogamia Heterogamy E1.0.0.0.0.0.5 Endogamia Endogamy E1.0.0.0.0.0.6 Sequentia reproductionis Reproductive sequence E1.0.0.0.0.0.7 Ovulatio Ovulation E1.0.0.0.0.0.8 Erectio Erection E1.0.0.0.0.0.9 Coitus Coitus; Sexual intercourse E1.0.0.0.0.0.10 Ejaculatio1 Ejaculation E1.0.0.0.0.0.11 Emissio Emission E1.0.0.0.0.0.12 Ejaculatio vera Ejaculation proper E1.0.0.0.0.0.13 Semen Semen; Ejaculate E1.0.0.0.0.0.14 Inseminatio Insemination E1.0.0.0.0.0.15 Fertilisatio Fertilization E1.0.0.0.0.0.16 Fecundatio Fecundation; Impregnation E1.0.0.0.0.0.17 Superfecundatio Superfecundation E1.0.0.0.0.0.18 Superimpregnatio Superimpregnation E1.0.0.0.0.0.19 Superfetatio Superfetation E1.0.0.0.0.0.20 Ontogenesis Ontogeny E1.0.0.0.0.0.21 Ontogenesis praenatalis Prenatal ontogeny E1.0.0.0.0.0.22 Tempus praenatale; Tempus gestationis Prenatal period; Gestation period E1.0.0.0.0.0.23 Vita praenatalis Prenatal life E1.0.0.0.0.0.24 Vita intrauterina Intra-uterine life E1.0.0.0.0.0.25 Embryogenesis2 Embryogenesis; Embryogeny E1.0.0.0.0.0.26 Fetogenesis3 Fetogenesis E1.0.0.0.0.0.27 Tempus natale Birth period E1.0.0.0.0.0.28 Ontogenesis postnatalis Postnatal ontogeny E1.0.0.0.0.0.29 Vita postnatalis Postnatal life E1.0.1.0.0.0.1 Mensurae embryonicae et fetales4 Embryonic and fetal measurements E1.0.1.0.0.0.2 Aetas a fecundatione5 Fertilization