An Integrated Approach to the Safety Assessment of Food Additives in Early Life
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Chapter 8: Food Additives
8 Food Additives Tanya Louise Ditschun and Carl K. Winter CONTENTS Introduction Food Additive Functionality Food Additive Regulations Generally Recognized as Safe (GRAS) The Delaney Clause Unintentional Additives Assessment of Food Safety` Specific Food Additives Under Scrutiny Saccharin Aspartame Hydrolysis Products of Aspartame Aspartic Acid Phenylalanine Methanol Diketopiperazine Marketing of Aspartame Erythrosine (FD & C Red #3) Olestra Anectodal Reports of Health Effects Due to Olestra Effects of Olestra on Nutrient Absorption Vitamin A Vitamin E Vitamin D Vitamin K Triglycerides Dietary Phytochemicals References © 2000 by CRC Press LLC Introduction Food additives have been used for centuries in food processing practices such as smoking and salting meat. Prior to the advent of refrigeration, food grown in the summer had to be preserved for the winter; salt, sugar, and vinegar were commonly used preservatives. The pursuits of explorers such as Marco Polo were often for food additives. Additives serve many roles and common uses include maintaining product consistency and pal- atability, providing leavening or control pH, enhancing flavor, and impart- ing color. A food additive can be defined in many ways. The Codex Alimentarius Commission, which develops international regulatory guidelines for food additives, provides the following definition of a food additive: Any substance not normally consumed as a food by itself, and not normal- ly used as a typical ingredient of the food, whether or not it has nutritive value, the intentional addition of which to food for a technological (includ- ing organoleptic) purpose in the manufacture, processing, preparation, treatment, packing, packaging, transport or holding of such food results, or may reasonably be expected to result, directly or indirectly, in it or its by-products becoming a component of or otherwise affecting the charac- teristics of such food. -
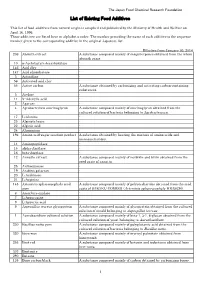
List of Existing Food Additives
The Japan Food Chemical Research Foundation List of Existing Food Additives This list of food additives from natural origin is complied and published by the Ministry of Health and Welfare on April 16, 1996. These additives are listed here in alphabetic order. The number preceding the name of each additive is the sequence number given to the corresponding additive in the original Japanese list. Effective from January 30, 2014 236 Absinth extract A substance composed mainly of sesquiterpenes obtained from the whole absinth grass. 10 α-Acetolactate decarboxylase - 146 Acid clay - 147 Acid phosphatase - 3 Actinidine - 56 Activated acid clay - 55 Active carbon A substance obtained by carbonizing and activating carbon-containing substances. 5 Acylase - 11 5'-Adenylic acid - 2 Agarase - 4 Agrobacterium succinoglycan A substance composed mainly of succinoglycan obtained from the cultured solution of bacteria belonging to Agrobacteriurn. 17 L-Alanine - 23 Alginate lyase - 22 Alginic acid - 24 Aluminium - 196 Amino acid-sugar reaction product A substance obtained by heating the mixture of amino acids and monosaccharides. 14 Aminopeptidase - 15 alpha-Amylase - 16 beta-Amylase - 12 Annatto extract A substance composed mainly of norbixin and bixin obtained from the seed coats of annatto. 25 Anthocyanase - 19 Arabino galactan - 20 L-Arabinose - 21 L-Arginine - 145 Artemisia sphaerocephala seed A substance composed mainly of polysaccharides obtained from the seed gum coats of SABAKU-YOMOGI (Artemisia sphaerocephala KRASCH). 6 Ascorbate oxidase - 7 L-Asparagine - 8 L-Aspartic acid - 9 Aspergillus terreus glycoprotein A substance composed mainly of glycoprotein obtained from the cultured solution of mould belonging to Aspergillus terreus . 1 Aureobasidium cultured solution A substance composed mainly of beta-1, 3-1, 6-glucan obtained from the cultured solution of yeast belonging to Aureobasidium . -

Investigation of Commodity Food Standards and Food Additives in Asia”(Ⅲ)
FY2011 Financial Supports for Promoting the “Sixth Industry” in Agriculture, Forestry and Fisheries and Rural Areas Creation and Promotion of the “Sixth Industry” for Pioneering the Future Overseas Business Support Project for Japanese Food Industry in East Asia “Investigation of Commodity Food Standards and Food Additives in Asia”(Ⅲ) Reported by : Hiroaki Hamano, International Life Sciences Institute (ILSI Japan) Investigation Ryoichi Akahoshi (Yakult Honsha Co., Ltd.) Collaborator : Yumi Asada (Unilever Japan Co.) Hiroshi Iwamoto (Morinaga Milk Industry Co., Ltd.) Youichiro Umeki (Danisco Japan Co.) Toshihisa Ohta (Yakult Honsha Co., Ltd.) Hiromi Ohta (Suntory Wellness Ltd.) Yoko Ogiwara (Ajinomoto Co., Inc. ASEAN Regional HQs) Satoru Kasai (Nihon Kraft Foods Limited) Kiyohisa Kaneko (Coca-Cola (Japan) Co., Ltd.) Kaori Kusano (Kirin Group Office Company, Ltd.) Yoshiharu Kuma (Kuma Consulting Engineer Office) Masanori Kohmura (Ajinomoto Co., Inc.) Yukio Suzuki (Schiff's Japan) Fumiko Sekiya (Takasago International Co.) Tomoko Takahashi (Nestle Japan Ltd.) Hisahiro Tatewaki (Kirin Holdings Company, Ltd.) Hidekazu Hosono (Suntory Business Expert Ltd.) Kensuke Watanabe (Suntory Business Expert Ltd.) Ryuji Yamaguchi (ILSI Japan) Hisami Shinohara (ILSI Japan) Shuji Iwata (ILSI Japan) Kazuo Sueki (ILSI Japan) ILSI Korea ILSI Focal Point in China ILSI Southeast Asia Region 1. Purpose of the Investigation In order to strengthen management practices and international competitiveness of Japanese food industry that is facing quantitative saturation and maturity in domestic market, it is necessary to address developing business in East Asian - 1 - regions where attractive market is forming due to increasing population and dynamically growing economy. In the past, Japanese food industry has been reluctant to develop new business in East Asia due to lack of information and understanding on food standards, methods of analysis, and conditions for use of food additives in the countries. -

Codex Alimentarius Commission FOOD and AGRICULTURE WORLD HEALTH ORGANIZATION ORGANIZATION of the UNITED NATIONS
codex alimentarius commission FOOD AND AGRICULTURE WORLD HEALTH ORGANIZATION ORGANIZATION OF THE UNITED NATIONS JOINT OFFICE: Via delle Terme di Caracalla 00100 ROME: Tel. 5797 Cables Foodagri ALINORM 78/12 JOINT FAO/WHO FOOD STANDARDS PROGRAMME CODEX ALIMENTARIUS COMMISSION Twelfth Session, April 1978 REPORT OF THE ELEVENTH SESSION OF THE CODEX COMMITTEE ON FOOD ADDITIVES The Hague, 31 May - 6 June 1977 TABLE OF CONTENTS Page Introduction, Appointment of Rapporteurs, Adoption of the Agenda 1 Appointment of Working Groups 1 Matters of Interest to the Committee 2 Consideration of the Report of the ad hoc Working Group on Flavours 5 Report of the ad hoc Working Group on Food Additive Intake 6 Endorsement of Food Additives in Codex Commodity Standards 7 Endorsement of Maximum Levels for Contaminants in Codex Commodity 11 Standards Consideration of Hydrolyzed Protein 12 List C of Food Additives 13 Establishment of Revised Codex List of Food Additives 13 Lists A and C 14 Advisory List of Additives in Soft Drinks 14 Consideration of Processing Aids 15 Consideration of the Report of the Working Group on Specifications 16 Specifications for Food Grade Salt 17 Revised Proposed Draft General Standard for the Labelling of Food Additives 17 when Sold as such Consideration of the Food Irradiation Process 18 Priority List for Food Additives 21 Note Concerning the Various ad hoc Working Groups 21 Future work 21 Other Business 21 Time and Place of Next Session 21 Closure of the Session 22 APENDICES Appendix I - List of Participants 22 Appendix II - Report -

E Number from Wikipedia, the Free Encyclopedia
E number From Wikipedia, the free encyclopedia E numbers are codes for substances which can be used as food additives for use within the European Union[1] and Switzerland (the "E" stands for "Europe").[2] They are commonly found on food labels throughout the European Union.[3] Safety assessment and approval are the responsibility of the European Food Safety Authority.[4] Having a single unified list for food additives was first agreed upon in 1962 with colours. In 1964, the directives for preservatives were added, 1970 for antioxidants and 1974 for the emulsifiers, stabilisers, thickeners and gelling agents.[5] Contents A solution of E101 riboflavin (also 1 Numbering scheme known as Vitamin B2) 2 Colloquial use 3 Classification by numeric range 4 Full list 4.1 E100–E199 (colours) 4.2 E200–E299 (preservatives) 4.3 E300–E399 (antioxidants, acidity regulators) 4.4 E400–E499 (thickeners, stabilizers, emulsifiers) 4.5 E500–E599 (acidity regulators, anti-caking Crystals of E621 Monosodium glutamate, a flavour enhancer agents) 4.6 E600–E699 (flavour enhancers) 4.7 E700–E799 (antibiotics) 4.8 E900–E999 (glazing agents and sweeteners) 4.9 E1000–E1599 (additional chemicals) 5 See also 6 Notes 7 External links Numbering scheme The numbering scheme follows that of the International Numbering System (INS) as determined by the Codex Alimentarius committee,[6] though only a subset of the INS additives are approved for use in the European Union as food additives. E numbers are also encountered on food labelling in other jurisdictions, including the Cooperation Council for the Arab States of the Gulf, Australia, New Zealand[7] and Israel. -

(1999) 719 Final WHITE PAPER on FOOD SAFETY
COMMISSION OF THE EUROPEAN COMMUNITIES Brussels, 12 January 2000 COM (1999) 719 final WHITE PAPER ON FOOD SAFETY TABLE OF CONTENTS EXECUTIVE SUMMARY.....................................................................................................3 CHAPTER 1: INTRODUCTION ...........................................................................................6 CHAPTER 2: PRINCIPLES OF FOOD SAFETY ..................................................................8 CHAPTER 3: ESSENTIAL ELEMENTS OF FOOD SAFETY POLICY: INFORMATION GATHERING AND ANALYSIS – SCIENTIFIC ADVICE .................................................10 CHAPTER 4: TOWARDS ESTABLISHING A EUROPEAN FOOD AUTHORITY...........14 CHAPTER 5: REGULATORY ASPECTS...........................................................................22 CHAPTER 6: CONTROLS ..................................................................................................29 CHAPTER 7: CONSUMER INFORMATION .....................................................................31 CHAPTER 8: INTERNATIONAL DIMENSION.................................................................34 CHAPTER 9: CONCLUSIONS ...........................................................................................36 ANNEX ...............................................................................................................................37 EXECUTIVE SUMMARY Assuring that the EU has the highest standards of food safety is a key policy priority for the Commission. This White Paper reflects this priority. A radical -

FSSAI Does Not Allow Use of Formalin for Fish Preservation July 18, 2018 in Goa News NT NETWORK
FSSAI does not allow use of formalin for fish preservation July 18, 2018 in Goa News NT NETWORK PANAJI Food Safety and Standards Authority of India, the autonomous body looking after food safety and regulations in the country, does not allow formaldehyde or formalin to be used as preservatives on fresh fish and fish products. The Food Safety and Standard (Food Products Standard and Food Additives) Regulation 2011 issued by the Union ministry of health and family welfare does not include formaldehyde under the list of food additives and preservatives for use in any food products. The notification, which is available on the FSSAI website, says the quantity of the additive added to fish shall be limited to the lowest possible level necessary to accomplish its desired effect. The limit is set between 30mg/kg to 100mg/kg. The food regulator allows the use of only potassium bisulphate, potassium sulphite, sodium metabisulphate, sodium sulphite, all are referred as sulphur dioxide, to be used as preservatives on selected fish products like frozen shrimps, lobster and fish fillets, salted fish, frozen finfish, canned finfish, canned shrimps, canned sardines, canned tuna, bonito and crab meat. The regulation also allows the manufacturers or producers of fish products to use antioxidants like ascorbic acid and also permit acidification which is another means of preserving fish and fish products by using citric acid and acetic acid in such quantity that the pH is not below 5. However, there is no mention of formaldehydes or its other most commonly known names such as methanal, methylene oxide, oxymethylene, methyl aldehyde, oxomethane, formic aldehyde, formol, fannoform, lysoform, morbicid acid, superlysoform and trioxane in the Food Safety and Standard (Food Products Standard and Food Additives) Regulation 2011. -

Food Additives
Food additives Department of Chemistry The Open University of Sri Lanka 1 Published by The Open University of Sri Lanka 2014 Food additives Introduction In previous lessons, we considered the chemistry of synthetic polymers, natural polymers and biomolecules such as carbohydrates, amino acids, proteins, fatty acids and lipids. In this lesson, we intend to study pros and cons of food additives. People around the world use natural and artificial food additives particularly to improve the taste and appearance of food without thinking of their effects on their health. Do you have the practice of checking the list of food additives given in the label when you buy food items? Centuries ago and even today people smoke fish/meat and add certain chemicals to them (e.g. salt) to improve their shelf life. Particularly in eastern countries, people add spices and indigenous herbs to food to improve its taste and colour. Some food varieties are seasonal (and abundant during the season) and are not available throughout the year. But preserved food is made available around the year, enabling us to enjoy food in different forms, even in places where it is not available or produced. Consumption of food additives may have certain health risks. Carcinogenesis (i.e. causation of cancers), hyperactivity in children, precipitation of allergies, and migraine are some known health risks associated with food additives. It is high time you knew more about what you eat. Let us examine the definition of a food additive. Food additives can be defined as chemical substances deliberately added to food, directly or indirectly, in known or regulated quantities, for purposes of assisting in the processing of food, preservation of food, or in improving the flavour, texture, or appearance of food. -

Evaluation of Certain Food Additives
WHO Technical Report Series 1014 Evaluation of certain food additives Eighty-sixth report of the Joint FAO/WHO Expert Committee on Food Additives The World Health Organization (WHO) was established in 1948 as a specialized agency of the United Nations serving as the directing and coordinating authority for international health matters and public health. One of WHO’s constitutional functions is to provide objective and reliable information and advice in the field of human health, a responsibility that it fulfils in part through its extensive programme of publications. The Organization seeks through its publications to support national health strategies and address the most pressing public health concerns of populations around the world. To respond to the needs of Member States at all levels of development, WHO publishes practical manuals, handbooks and training material for specific categories of health workers; internationally applicable guidelines and standards; reviews and analyses of health policies, programmes and research; and state-of-the-art consensus reports that offer technical advice and recommendations for decision-makers. These books are closely tied to the Organization’s priority activities, encompassing disease prevention and control, the development of equitable health systems based on primary health care, and health promotion for individuals and communities. Progress towards better health for all also demands the global dissemination and exchange of information that draws on the knowledge and experience of all WHO Member States and the collaboration of world leaders in public health and the biomedical sciences. To ensure the widest possible availability of authoritative information and guidance on health matters, WHO secures the broad international distribution of its publications and encourages their translation and adaptation. -

Food Additives Regulation (EC) Nº 1333/2008 on Food Additives
Food Additives Regulation (EC) Nº 1333/2008 on Food Additives Ana Oliveira [email protected] 10-10-2017 LEGAL FRAMEWORK Food Improvement Agent Package (FIAP) Package includes 4 regulations: • Regulation (EC) Nº 1331/2008 Common approval procedure for food additives, food enzymes and food flavourings • Regulation (EC) Nº 1332/2008 Food Enzymes • Regulation (EC) Nº 1333/2008 Food Additives • Regulation (EC) Nº 1334/2008 Flavourings repealed and progressively replaced a number of directives and regulations Regulations vs Directives • Immediate implementation as law in all Member States • Avoids different national interpretations LEGAL FRAMEWORK Regulation (EC) Nº 1333/2008 Subject matter (article 1) lays down rules on food additives used in foods Purpose (article 1) • ensure the effective functioning of the internal market • ensuring a high level of protection of human health and a high level of consumer protection, including the protection of consumer interests and fair practices in food trade Tools (article 1) • Community lists of approved food additives (Annexes II and III); • conditions of use of food additives in foods, including in food additives, in food enzymes and in food flavourings • rules on the labelling of food additives sold as such LEGAL FRAMEWORK Regulation (EC) Nº 1333/2008 Scope (article 2) • Food additives Does not apply (unless they are used as food additives) (article 2) • processing aids • substances used for the protection of plants and plant products • substances added to foods as nutrients; • substances used -

Food Additive Requirements Morocco
THIS REPORT CONTAINS ASSESSMENTS OF COMMODITY AND TRADE ISSUES MADE BY USDA STAFF AND NOT NECESSARILY STATEMENTS OF OFFICIAL U.S. GOVERNMENT POLICY Voluntary - Public Date: 8/31/2018 GAIN Report Number: MO1841 Morocco Post: Rabat Food Additive Requirements Report Categories: FAIRS Subject Report Sanitary/Phytosanitary/Food Safety Retail Foods Food Processing Ingredients SP2 - Prevent or Resolve Barriers to Trade that Hinder U.S. Food and Agricultural Exports Approved By: Morgan Haas Prepared By: FAS/Rabat Report Highlights: This report contains an unofficial translation of Order No. 1795-14, which sets out the list and limits of food additives authorized for use in primary products and food products, as well as the indications that their packaging must bear. For food additives not mentioned but which are considered by Codex Alimentarius as food additives, their presence is permitted within the limits provided by Codex Alimentarius. Morocco has not yet notified this measure to the WTO. Joint Order of the Minister of Agriculture and Maritime Fisheries and the Minister of Health No. 1795-14 of 14 rejeb 1435 (May 14, 2014) setting out the list and limits of food additives authorized for use in primary products and food products, as well as the indications that their packaging must bear. (BO No. 6322bis of 01/01/2015, page 425) THE MINISTER OF AGRICULTURE AND MARINE FISHERIES, THE MINISTER OF HEALTH, Considering Decree n ° 2-10-473 of 7 chaoual 1432 (September 6th, 2011) taken for the application of certain provisions of the Law n ° 28-07 relating to the safety of foodstuffs, in particular its article 53, 3), ORDER: FIRST ARTICLE. -

Safety Evaluation of Certain Food Additives and Contaminants / Prepared by the Seventy-Fourth Meeting of the Joint FAO/WHO Expert Committee on Food Additives (JECFA)
WHO FOOD Safety evaluation of ADDITIVES certain food additives SERIES: 65 and contaminants Prepared by the Seventy-fourth meeting of the Joint FAO/ WHO Expert Committee on Food Additives (JECFA) World Health Organization, Geneva, 2012 WHO Library Cataloguing-in-Publication Data Safety evaluation of certain food additives and contaminants / prepared by the Seventy-fourth meeting of the Joint FAO/WHO Expert Committee on Food Additives (JECFA). (WHO food additives series ; 65) 1.Food additives - toxicity. 2.Food contamination. 3.Flavoring agents - analysis. 4.Flavoring agents - toxicity. 5.Risk assessment. I.Joint FAO/WHO Expert Committee on Food Additives. Meeting (74th : 2011 : Rome, Italy). II.World Health Organization. III.Series. ISBN 978 92 4 166065 5 (NLM classification: WA 712) ISSN 0300-0923 © World Health Organization 2012 All rights reserved. Publications of the World Health Organization are available on the WHO web site (www.who.int) or can be purchased from WHO Press, World Health Organization, 20 Avenue Appia, 1211 Geneva 27, Switzerland (tel.: +41 22 791 3264; fax: +41 22 791 4857; e-mail: [email protected]). Requests for permission to reproduce or translate WHO publications—whether for sale or for non-commercial distribution—should be addressed to WHO Press through the WHO web site (www.who.int/about/licensing/copyright_form/en/index.html). The designations employed and the presentation of the material in this publication do not imply the expression of any opinion whatsoever on the part of the World Health Organization concern ing the legal status of any country, territory, city or area or of its authorities, or concern ing the delimitation of its frontiers or boundaries.