Domestic and Import Food Additives and Color Additives
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Food Allergen Management in Foodservice – a Best Practice Guideline
national allergy strategy Food Allergen Management in Foodservice A BEST PRACTICE GUIDELINE Developed by Statewide Foodservices Qld. Health This work is licensed under a Creative Commons Attribution Non-Commercial V4.0 International licence. To view a copy of this Block 7 Level 7 Royal Brisbane & Women’s Hospital, licence, visit https://creativecommons.org/licenses/by-nc/4.0/deed.en Butterfield St., Herston Qld 4029 You are free to copy, communicate and adapt the work for non- Ph. (07) 3646 2288 commercial purposes, as long as you attribute the State of Queensland (Queensland Health) and comply with the licence terms. [email protected] For copyright permissions beyond the scope of this licence contact: Intellectual Property Officer, Queensland Health, © State of Queensland (Queensland Health) 2018 email [email protected], phone (07) 3708 5069. PAGE 1 FOOD ALLERGEN MANAGEMENT IN FOODSERVICE – A BEST PRACTICE GUIDELINE Background Standards Added Sulphites in concentrations of 10 mg/kg or more In managing food allergies in health care foodservices there Cereals containing gluten and their products, namely, wheat, rye, barley, oats and spelt and their hybridised strains are overarching practices that are required as per the Food Crustacea and their products Standards Code (FSANZ) that will inform and support the Egg and egg products process of identifying, assessing, managing and auditing Fish and fish products, except for isinglass derived from swim bladders and the risk of food allergies in the food service. used as a clarifying agent in beer and wine Milk and milk products Peanuts and peanut products These include – Food Standards Australia & New Zealand (Chapter 1 – Food Allergen Labelling - A food allergy occurs when a person’s immune system Sesame seeds and sesame seed products reacts to allergens that are harmless to other people. -

Outline What Are What Are Processing a Processing Aids?
The Issue of Undeclared Ingredients in Halal ﻗﺿﻳﺔ اﻟﻣﻛوﻧﺎت ﻏﻳر اﻟﻣﻌﻠﻧﺔ ﻓﻲ اﻷﻏذﻳﺔ ا ﻟ ﻣ ﺟ ﻬ زة اﻟﺣﻼﻝ؟ Food Processing اﻟﺗرﻛﻳز ﻋﻠﻰ ﻣﻌﺎﻟﺟﺎت اﻟﺗﺻﻧﻳﻌﻳﺔ A Focus on Processing Aids أ.د. ﻣﻳﺎن ﻣﺣﻣد ﻧدﻳم رﻳﺎض .Mian N. Riaz, Ph.D ﻣدﻳر أﺑﺣﺎث اﻟﺑروﺗﻳن، ﺟﺎﻣﻌﺔ ﺗﻛﺳﺎس أﻳﻪ أﻧد أم، ﺗﺎﻣو، اﻟوﻻﻳﺎت Food Protein R&D Center; Texas A&M University اﻟﻣﺗﺣدة اﻷﻣرﻳﻛﻳﺔ College Station, Texas USA ورشة الحﻻل اﻷولى First Halal Workshop 11 مايو May, 2014 2014 11 معھد الكويت لﻷبحاث العلمية Kuwait Institute for Scientific دولة الكويت Research, State of Kuwait Outline What are Processing AAids?ids? Processing aids and Hidden Ingredients Processing aids and Hidden Ingredients What are processing aids?aids? Processing aids are substances that are approved by both the Sources of processing aidsaids Food and Drug Administration Why use processing aids and hidden (FDA) and the U.S. Department ingredients? of Agriculture (USDA) Are All hidden ingredients are Halal? They are used in the production Examples of hidden ingredients of a variety of foods – meat, Control of Halal food processing aid poultry, produce, etc., and are and hidden ingredients not present in any significant amount in the finished product What are Processing Aids? What are Processing aids? Processing Aids and Hidden Ingredients Processing Aids and Hidden Ingredients The use of food Both the FDA and USDA recognize three situations in which a processing aid has manufacturing substance is deemed to be a processing aid: become more prominent in recent 11.. When substances are added to a food during processing years, due to the but subsequently removed before the food reaches its finished increased production form (example activated charcoals which filter out impurities) of prepared, processed, and convenience foods What are Processing aids? What are Processing aids? Processing Aids and Hidden Ingredients Processing Aids and Hidden Ingredients 2. -

Five Keys to Safer Food Manual
FIVE KEYS TO SAFER FOOD MANUAL DEPARTMENT OF FOOD SAFETY, ZOONOSES AND FOODBORNE DISEASES FIVE KEYS TO SAFER FOOD MANUAL DEPARTMENT OF FOOD SAFETY, ZOONOSES AND FOODBORNE DISEASES INTRODUCTION Food safety is a significant public health issue nsafe food has been a human health problem since history was first recorded, and many food safety Uproblems encountered today are not new. Although governments all over the world are doing their best to improve the safety of the food supply, the occurrence of foodborne disease remains a significant health issue in both developed and developing countries. It has been estimated that each year 1.8 million people die as a result of diarrhoeal diseases and most of these cases can be attributed to contaminated food or water. Proper food preparation can prevent most foodborne diseases. More than 200 known diseases are transmitted through food.1 The World Health Organization (WHO) has long been aware of the need to educate food handlers about their responsibilities for food safety. In the early 1990s, WHO developed the Ten Golden Rules for Safe Food Preparation, which were widely translated and reproduced. However, it became obvious that something simpler and more generally applicable was needed. After nearly a year of consultation with food safety expertsandriskcommunicators, WHOintroducedtheFive KeystoSaferFoodposterin2001.TheFive Keys toSaferFoodposterincorporatesallthemessagesoftheTen Golden Rules for Safe Food Preparation under simpler headings that are more easily remembered and also provides more details on the reasoning behind the suggested measures. The Five Keys to Safer Food Poster The core messages of the Five Keys to Safer Food are: (1) keep clean; (2) separate raw and cooked; (3) cook thoroughly; (4) keep food at safe temperatures; and (5) use safe water and raw materials. -

Suitability of Food Additives: Combating Chemophobia and Consumer Misunderstanding
Suitability of Food Additives: Combating Chemophobia and Consumer Misunderstanding Priscilla Zawislak Ashland, Inc. International Food Additives Council Safety of Food Additives • Food additives have been used safely for decades. • Food additives are thoroughly studied, including extensive toxicological testing and consideration of quality and specifications, before they are approved for use in food. • Testing includes short-term and long-term toxicity studies, including carcinogenicity studies with a built in safety factor to account for uncertainties. Suitability of Food Additives • Food additives afford us the convenience and enjoyment of a wide variety of appetizing, nutritious, fresh and palatable foods. • Food additives are critical to safe and nutritious foods and beverages. • Food additives used for technical purposes in finished foods fall into four main categories: Support nutrition delivery Support the maintenance of food quality and freshness Aid in processing and preparation of foods Make foods appealing to the consumer Feeding the World • Global Population Growth: 7 billion by 2010 9 billion by 2050 • That is 75 million more people to feed each year. • Almost 1 billion don’t have enough food today. • Food additives provide a solution to keep food safe and minimize food waste. Food Additives Have Many Technological Functions in Foods • Codex International Numbering System (INS) lists 23 functional classes for food additives, such as gelling agent, emulsifier, anticaking agent, etc. • System is hierarchical in that each of the 23 functional classes has sub-classes with additional functions. Good Manufacturing Practices (GMP) • GMPs state lowest level of food additive necessary to achieve technological function should be used in finished foods and beverages. -

A Simple Fluorimetric Method to Determine Sudan I Dye in Spices
Karadeniz Chem. Sci. Tech., 2017, 01, EA.1 - EA.4 Extended Abstract (Fulltext available in Turkisha) A Simple fluorimetric method to determine Sudan I dye in spices Osman Can Çağılcı, Abidin Gümrükçüoğlu, Hakan Alp, Elvan Vanlı, Ümmühan Ocak*, Miraç Ocak Department of Chemistry, Faculty of Sciences, Karadeniz Technical University, 61080 Trabzon, Turkey ABSTRACT Keywords: A simple method was developed to determine Sudan I, a banned food dye, in various spices by using its intrinsic fluorescence Sudan I dye, property. The proposed method was used in the determination of red pepper, sumac and cumin samples to which were added fluorescence method, Sudan I dye after being obtained from the local sources. The accuracy of method has been verified by spike and recovery fluorimetry, experiments. The recovery values of Sudan I were found in the range of 96.6% - 97.6% for pepper, sumac and cumin. A kind of standard addition method was used to increase the effect of matrix match. Detection limits were 1.0, 0.2 and 0.3 mg/L for red pepper, red pepper, sumac and cumin, respectively. When compared with the methods in the literature, the proposed method is simple, sumac, cumin environmentally friendly and low cost to determine the quantity of Sudan I in spices such as pepper, sumac and cumin. 1. Introduction calcein complex used to determine Sudan I [28]. Chen et al. proposed a method based on the use of polyethylenimine-coated copper Sudan dyes are synthetic azo dyes, used in textile and cosmetic nanoparticles [29], while Ling et al. reported the use of products. -

Japan Japan Invites Comments for Genome-Edited Foods Handling Procedure
THIS REPORT CONTAINS ASSESSMENTS OF COMMODITY AND TRADE ISSUES MADE BY USDA STAFF AND NOT NECESSARILY STATEMENTS OF OFFICIAL U.S. GOVERNMENT POLICY. Voluntary - Public Date: 7/5/2019 GAIN Report Number: JA9096 Japan Post: Tokyo Japan Invites Comments for Genome-Edited Foods Handling Procedure Report Categories: Biotechnology and Other New Production Technologies Agricultural Situation Grain and Feed Approved By: Barrett Bumpas Prepared By: Suguru Sato Report Highlights: On June 27, 2019, the Ministry of Health, Labour and Welfare (MHLW) published a draft of the Handling Procedure of Foods and Food Additives Derived from Genome Editing Technology under Food Sanitation Act. Comments must be submitted in Japanese via an online system, mail, or fax by Friday, July 26, 2019. A WTO-SPS notification is unlikely since there are no legal changes. General Information: MHLW released a genome-edited food policy on March 27, 2019 (JA9050), however, internal discussion to establish the specific guideline for the handling of genome edited foods has continued. On June 27, 2019, MHLW published a draft of the Handling Procedure of Foods and Food Additives Derived from Genome Editing Technology under Food Sanitation Act. Comments must be submitted in Japanese via an online system, mail, or fax by Friday, July 26, 2019. A WTO-SPS notification is unlikely since there are no legal changes. How to Submit Comments 1. Comments on regulatory proposals by the Japanese Government can be sent electronically via the “e-Gov” system. The site can be found at: https://search.e- gov.go.jp/servlet/Public?CLASSNAME=PCMMSTDETAIL&id=495190105&Mode=0 1. -

The Food Wars Thesis
.CHAPTER 1 THE FOOD WARS THESIS 'Ifyouknow before you look, you cannot see for knowing.' Sir Terry Frost RA (British artist 1915-2003) CORE ARGUMENTS Different visions for the future of food are shaping the potential for how food will be produced and marketed. Inevitably, there will be policy choices - for the state, the corporate sector and civil society. Human and environ mental health needs to be at the heart of these choices. Three broad conceptual frameworks or 'paradigms' pro pose the way forward for food policy, the food economy and health itself. All make claims to raise production and to deliver health benefits through food. The challenge for policy-makers is how to sift through the evidence and to give a fair hearing to a range of choices. This process is sometimes difficult because the relationship betweenevid ence and policy is not what it seems. The world of food is on the cusp of a far-reaching transition. INTRODUCTION The world is producingmore food than ever to feed more mouths than ever,' For the better off there are more food and beverage product choices than it is possible to imagine - globally 25,000 products in the average supermarket and more than 20,000 new packaged foods and beverages in 2002 alone.? Yet for many people there is a general feeling of unease and mistrust about the 12 FOOD WARS future of our food supply. Food and problems associated with producing and consuming food generate political and policy crises and are regular fodder for media coverage. In addition, along with the food production successes of the past 40 years in reducing famine, hunger continues hand in hand with excess. -

Peanut Butter Consumption and Hepatocellular Carcinoma in Sudan
PEANUTBUTTE RCONSUMPTIO NAN DHEPATOCELLULA R CARCINOMAI N SUDAN Ragaa ElHad iOme r Promotor: Prof.dr.ir. F.J.Ko k Wageningen Universiteit Co-promotor: Dr.ir.P .va n 't Veer Wageningen Universiteit Samenstelling Prof.dr. J.H.Koema n Promotiecommissie: Wageningen Universiteit Prof.dr.ir. F.E.va n Leeuwen Vrije Universiteit Amsterdam Prof.dr. C.E. West KatholiekeUniversitei t Nijmegen Wageningen Universiteit Prof.dr. J. Jansen Katholieke Universiteit Nijmegen PEANUT BUTTER CONSUMPTION AND HEPATOCELLULAR CARCINOMA IN SUDAN Ragaa ElHad iOme r Proefschrift terverkrijgin g van degraa dva n doctor opgeza gva n derecto r magnificus van Wageningen Universiteit Prof.dr.ir. L.Speelma n in het openbaar te verdedigen opmaanda g 12maar t 2001 desnamiddag st e 16.00 uur in deAul a .QDlWO The research described in this thesis was funded by the Sudanese Standards and Meteorology Organisation (SSMO), Wageningen University and the University of Khartoum. Further support was obtained from the RIKILT-DLO Institute in Wageningen and the Forestry National Corporation in Khartoum. Financial support for the printing of this thesis was obtained from the Dr.Judit h Zwartz Foundation, Wageningen, The Netherlands. OmerE l Hadi, Ragaa Peanut butter consumption and hepatocellular carcinoma in Sudan: acase-contro l study Thesis Wageningen University - With ref. - With summary in Arabic ISBN 90-5808-366-7 Printing: Grafisch bedrijf Ponsen and Looijen B.V.,Wageningen , The Netherlands © Omer 2001 To thespirit of mylovely father Abstract PEANUT BUTTERCONSUMPTIO N AND HEPATOCELLULAR CARCINOMA IN SUDAN Ph.D. thesis by Ragaa El Hadi Omer, Division of Human Nutrition and Epidemiology, Wageningen University, Wageningen, TheNetherlands. -

Chapter 8: Food Additives
8 Food Additives Tanya Louise Ditschun and Carl K. Winter CONTENTS Introduction Food Additive Functionality Food Additive Regulations Generally Recognized as Safe (GRAS) The Delaney Clause Unintentional Additives Assessment of Food Safety` Specific Food Additives Under Scrutiny Saccharin Aspartame Hydrolysis Products of Aspartame Aspartic Acid Phenylalanine Methanol Diketopiperazine Marketing of Aspartame Erythrosine (FD & C Red #3) Olestra Anectodal Reports of Health Effects Due to Olestra Effects of Olestra on Nutrient Absorption Vitamin A Vitamin E Vitamin D Vitamin K Triglycerides Dietary Phytochemicals References © 2000 by CRC Press LLC Introduction Food additives have been used for centuries in food processing practices such as smoking and salting meat. Prior to the advent of refrigeration, food grown in the summer had to be preserved for the winter; salt, sugar, and vinegar were commonly used preservatives. The pursuits of explorers such as Marco Polo were often for food additives. Additives serve many roles and common uses include maintaining product consistency and pal- atability, providing leavening or control pH, enhancing flavor, and impart- ing color. A food additive can be defined in many ways. The Codex Alimentarius Commission, which develops international regulatory guidelines for food additives, provides the following definition of a food additive: Any substance not normally consumed as a food by itself, and not normal- ly used as a typical ingredient of the food, whether or not it has nutritive value, the intentional addition of which to food for a technological (includ- ing organoleptic) purpose in the manufacture, processing, preparation, treatment, packing, packaging, transport or holding of such food results, or may reasonably be expected to result, directly or indirectly, in it or its by-products becoming a component of or otherwise affecting the charac- teristics of such food. -

Artificial Food Colours and Children Why We Want to Limit and Label Foods Containing the ‘Southampton Six’ Food Colours on the UK Market Post-Brexit
Artificial food colours and children Why we want to limit and label foods containing the ‘Southampton Six’ food colours on the UK market post-Brexit November 2020 FIRST STEPS NUTRITIONArtificial food coloursTRUST and children: page Artificial food colours and children: Why we want to limit and label foods containing the‘Southampton Six’ food colours on the UK market post-Brexit November 2020 Published by First Steps Nutrition Trust. A PDF of this resource is available on the First Steps Nutrition Trust website. www.firststepsnutrition.org The text of this resource, can be reproduced in other materials provided that the materials promote public health and make no profit, and an acknowledgement is made to First Steps Nutrition Trust. This resource is provided for information only and individual advice on diet and health should always be sought from appropriate health professionals. First Steps Nutrition Trust Studio 3.04 The Food Exchange New Covent Garden Market London SW8 5EL Registered charity number: 1146408 First Steps Nutrition Trust is a charity which provides evidence-based and independent information and support for good nutrition from pre-conception to five years of age. For more information, see our website: www.firststepsnutrition.org Acknowledgements This report was written by Rachael Wall and Dr Helen Crawley. We would like to thank Annie Seeley, Sarah Weston, Erik Millstone and Anna Rosier for their help and support with this report. Artificial food colours and children: page 1 Contents Page Executive summary 3 Recommendations -
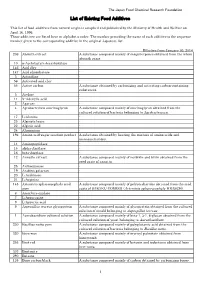
List of Existing Food Additives
The Japan Food Chemical Research Foundation List of Existing Food Additives This list of food additives from natural origin is complied and published by the Ministry of Health and Welfare on April 16, 1996. These additives are listed here in alphabetic order. The number preceding the name of each additive is the sequence number given to the corresponding additive in the original Japanese list. Effective from January 30, 2014 236 Absinth extract A substance composed mainly of sesquiterpenes obtained from the whole absinth grass. 10 α-Acetolactate decarboxylase - 146 Acid clay - 147 Acid phosphatase - 3 Actinidine - 56 Activated acid clay - 55 Active carbon A substance obtained by carbonizing and activating carbon-containing substances. 5 Acylase - 11 5'-Adenylic acid - 2 Agarase - 4 Agrobacterium succinoglycan A substance composed mainly of succinoglycan obtained from the cultured solution of bacteria belonging to Agrobacteriurn. 17 L-Alanine - 23 Alginate lyase - 22 Alginic acid - 24 Aluminium - 196 Amino acid-sugar reaction product A substance obtained by heating the mixture of amino acids and monosaccharides. 14 Aminopeptidase - 15 alpha-Amylase - 16 beta-Amylase - 12 Annatto extract A substance composed mainly of norbixin and bixin obtained from the seed coats of annatto. 25 Anthocyanase - 19 Arabino galactan - 20 L-Arabinose - 21 L-Arginine - 145 Artemisia sphaerocephala seed A substance composed mainly of polysaccharides obtained from the seed gum coats of SABAKU-YOMOGI (Artemisia sphaerocephala KRASCH). 6 Ascorbate oxidase - 7 L-Asparagine - 8 L-Aspartic acid - 9 Aspergillus terreus glycoprotein A substance composed mainly of glycoprotein obtained from the cultured solution of mould belonging to Aspergillus terreus . 1 Aureobasidium cultured solution A substance composed mainly of beta-1, 3-1, 6-glucan obtained from the cultured solution of yeast belonging to Aureobasidium . -

Global Regulations of Food Colors Each Region Has Its Own Definitions of What Constitutes a Color Additive, with Related Use Requirements and Restrictions
Global Regulations of Food Colors Each region has its own definitions of what constitutes a color additive, with related use requirements and restrictions. Sue Ann McAvoy Sensient Colors LLC t is said, we eat with our eyes . Since antiq - food colors was soon recognized as a threat Iuity, humans have used the color of a to public health. Of concern was that some food to discern its quality. Color provides of the substances were known to be poi - a way to judge ripeness, perceive flavor and sonous and were often incorporated to hide assess quality of food. poor quality, add bulk to foods and to pass Ancient civilizations introduced color off imitation foods as real. into their foods. The ancient Egyptians col - On February 1, 1899, the executive com - ored their food yellow with saffron, and the mittee of the National Confectioners’ Asso - ancient Mayans used annatto to color their ciation published an official circular which Sue Ann McAvoy is food orange-red. Wealthy Romans ate bread was “to throw light upon the vexed question global regulatory scien - that had been whitened by adding alum to of what colors may be safely used in confec - tist for Sensient Food the flour. Color could be used to enhance tionery” as “there may at times be a doubt in Colors LLC. She has worked at Sensient the mind of the honest confectioner as to the physical appearance of the product. since 1979. However, if it made the food appear to be which colors, flavors, or ingredients he may of better quality than it was, that was con - safely use and which he may reject.” This list sidered a deceitful practice.