Chapter 8: Food Additives
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Food, Dietary Supplement & Cosmetics Regulatory Update
Issue Number 2 | March 2014 Top Stories FDA Extends Comment Period for Proposed Rule on Intentional Adulteration and Accompanying Risk Analysis On March 24, FDA announced that it would extend the comment period for its proposed rule on intentional adulteration and the accompanying risk assessment document. The proposed rule and the risk assessment were published in the December 24, 2013 Federal Register with a 100-day comment period. However, in response to stakeholder concerns that more time is needed to evaluate the proposed rule, due to the inherent complexity and unique nature of food defense issues, FDA granted a 90-day extension to June 30, 2014. Because the risk assessment is directly related to the proposed rule, FDA also extended the comment period for the risk assessment document. The proposed rule, which would not apply to farms or animal food, would require every food facility to have a written food defense plan addressing "significant vulnerabilities" in its particular food production processes. In addition, facilities would be required to identify and implement strategies to address the identified vulnerabilities, establish monitoring procedures and corrective actions, verify the system is working, and ensure training of personnel assigned to each vulnerable area. Comments due June 30 to Docket FDA-2013-N-1563. FDA Seeks Input on Information to be Submitted to FDA's Reportable Food Registry and Used to Notify Consumers in Grocery Stores On March 26, FDA issued an advance notice of proposed rulemaking seeking input to assist FDA in implementing section 211 of the Food Safety Modernization Act ("FSMA"). Section 211 added new provisions to the Portable Food Registry requirements of the Federal Food, Drug, and Cosmetic Act. -

C O M M E N T A
Calorie Control COMMENTARY Spring/Summer 1999 Vol. 21, No.1 Experts Denounce Aspartame Misinformation Circulating The Berkeley on the Internet Wellness Letter pointed out that reputable multiple on't believe the rumors -- widely spread on the Internet -- sclerosis organi- Dthat aspartame ... causes not only multiple sclerosis, but also lupus, Alzheimer's and Parkinson's disease, zations and other diabetes, Gulf War syndrome, and brain tumors." -- health/medical April 1999 University of California, Berkeley organizations Wellness Letter. The Berkeley Wellness Letter is the latest media have denounced source to issue a salvo against misinformation about the rumors, and aspartame circulating on the Internet. that "aspartame "Like other recent Internet-based health 'alerts,' this has been more one is designed to scare the pants off you -- Internet terror- ism," the front page article notes, adding that the document intensively stud- circulated via e-mail by an unidentified source is "packed with Administration, American Medical Association and the ied than almost gobbledygook, strange anecdotes, misused jargon, mysterious World Health Organization, have concluded that aspar- any other food doctors selling quack theories, and impressive-sounding orga- tame is safe. The American Diabetes Association also nizations that may or may not exist." recently issued a position statement proclaiming the additive." The article pointed out that reputable multiple sclerosis safety of aspartame. organizations and other health/medical organizations have Specifically, -

0 L E S T R A
0 L E S T R A %..,X:- :.:-:X- ... X:: N. - ... : ::::::: N:. ... ::-N- ". :- ::: --:--.:.,:...:. X:- ,au:i.1 :.: ,:::. ..: :.. .::. ..X :::. :X ..:: ..... .:.: :.. .... :'. ":.: :-- -X::.:.::. :- ,::::: :: :::-" ....:... :4 ..::: :::::- ... --::: ..::- ::..:4:. .. -:::...::. :.%.:.. :::.: :Z, :::. N..-::....:::: ..N..." Y4 .:4:.. %1:4.1".: ..:::.. --::-::- :. --. :::: ,:::., :::.- ;::..........:-..1 ::,. :::t :... ..N-4,--41 .::N 2... :..:::: ":. :X----.-::: ..: ...I.:: ..:: :. .'. "". :fill,q :..,.. -:.:: :.:::: .X::- ,::. ..1.:.:..:...i.!-,,,..:, .:.::::::.:::: :::.. Nz .-.::. :::. .,"::.,,.. :......7 :4. ::.- :::..::::f::% -,-.,:.X:::-.: ..... .X::%:--::x..:- .::.4,:....:it...-.. -::: I::I:::....:---::":..... .:t:.. ::... .1-::::.;-":::::...:...:N..:P,f,.,:.;::: .:... ::::W...,--.. 4- ..::.X-:,:i.::: ..i.:4il..:..:..:,, ::xv,--w.....X1....,:: :-:-: :-.:: ::: !.m.,g,% .. .%.::.....1."::. I.:... .:. .M,:.: :.:..,: .:.4: :-. -X::.-..:...:. ..:!."..%: "::: -:X. :::.,. NX, .: .:., ....I:.- .....,:....-:. ...-..:: .:., :4. :........ M: :::!,-":W::.-Xx..::.-..-:..........1.:..,:.:. :::.. ... ::...-.., ...........I::: 0. :....:r:: ......-..,..,.:: 1: '--:."-.. :m: .::::..:. 'N:.'zi:....... I%... :I::.: I.......': N::.,::::. :4z :4,F:. :: :::..:.- :--:....::....x. ......... -1:......1:x: .,,:: ::!. :: :::. ...4'......-......,.... ::. ,-:::.-::: .: .... :::.n:.:- .Z::.. .:---- ::-.....:.:'........" :- :- :. -:- .::... ..:. -:::...: .-:-::!:.-I:::.::%:,z-:::::-%XX ,.:..........I..... .N.. --g-., .... :..-....., "-::: .:..:::.....:.:. .:.......-,.-h, -

LINC Foods Value Added Guidelines General Guidance: Must Be Made
LINC Foods Value Added Guidelines General Guidance: Must be made by farmers or processors with a food processor license. Ingredients will be organic if available and the cost is not significantly higher than non-organic options. Examples of organic ingredients: coconut, many seeds, rice, dried fruits, sweeteners except honey, extracts, flours, oats, olive oil, coconut oil, canola oil. Examples of non-organic ingredients: pectin, peanut-free tree nuts, dates, honey, certified GF oats, certified GF flours, vinegar, grapeseed oil. Flours: No bleached flour. Produce: Fresh produce must be organically grown and local. Dried produce ingredients must be organic and through LINC where practical. Sweeteners: Sugars and syrups must be organic. Honey must be local and raw (never heated above 120 degrees). Flavorings and colors: Use only “pure”, “natural”, or “organic compliant” flavorings and colors. No artificial flavorings or colors. Fats: No trans fats or artificial fats (for example Olestra). The LINC application should include copy of State and Health Department license or certifications. A member is required to inform LINC if there is a license change, kitchen change, etc. Justification for LINC Sponsoring Sales of Locally-Made Value-Added Products While recognizing the Value-Added foods are one step up the food chain from fresh produce and local unprocessed meats, it is also recognized that all foods we make require non-local inputs, and that even meats and grains go through value-added steps – sometimes off the farm (for example, malted barley). The advantages of selling locally produced products include: • They utilize local labor in production, • Finished goods do not need to be shipped far, • They can be made without as many preservatives, because the time period between manufacture and consumption can be shortened, • They increase LINC income. -
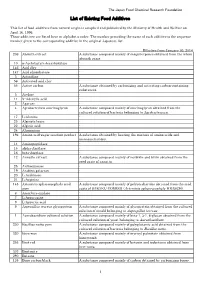
List of Existing Food Additives
The Japan Food Chemical Research Foundation List of Existing Food Additives This list of food additives from natural origin is complied and published by the Ministry of Health and Welfare on April 16, 1996. These additives are listed here in alphabetic order. The number preceding the name of each additive is the sequence number given to the corresponding additive in the original Japanese list. Effective from January 30, 2014 236 Absinth extract A substance composed mainly of sesquiterpenes obtained from the whole absinth grass. 10 α-Acetolactate decarboxylase - 146 Acid clay - 147 Acid phosphatase - 3 Actinidine - 56 Activated acid clay - 55 Active carbon A substance obtained by carbonizing and activating carbon-containing substances. 5 Acylase - 11 5'-Adenylic acid - 2 Agarase - 4 Agrobacterium succinoglycan A substance composed mainly of succinoglycan obtained from the cultured solution of bacteria belonging to Agrobacteriurn. 17 L-Alanine - 23 Alginate lyase - 22 Alginic acid - 24 Aluminium - 196 Amino acid-sugar reaction product A substance obtained by heating the mixture of amino acids and monosaccharides. 14 Aminopeptidase - 15 alpha-Amylase - 16 beta-Amylase - 12 Annatto extract A substance composed mainly of norbixin and bixin obtained from the seed coats of annatto. 25 Anthocyanase - 19 Arabino galactan - 20 L-Arabinose - 21 L-Arginine - 145 Artemisia sphaerocephala seed A substance composed mainly of polysaccharides obtained from the seed gum coats of SABAKU-YOMOGI (Artemisia sphaerocephala KRASCH). 6 Ascorbate oxidase - 7 L-Asparagine - 8 L-Aspartic acid - 9 Aspergillus terreus glycoprotein A substance composed mainly of glycoprotein obtained from the cultured solution of mould belonging to Aspergillus terreus . 1 Aureobasidium cultured solution A substance composed mainly of beta-1, 3-1, 6-glucan obtained from the cultured solution of yeast belonging to Aureobasidium . -

Olestra, Also Known As Sucrose Polyester, Is a Heterogeneous Synthetic Fat Substitute Consisting Primarily of Hexa-, Hepta
Olestra, also known as sucrose polyester, is a heterogeneous synthetic fat substitute consisting primarily of hexa -, hepta -, and octaesters formed by the reaction of sucrose with fatty acids derived form edible oil sources, including soybean, corn, and cot tonseed.(1) Olestra shares many of the physical properties of traditional dietary fats, including texture, palatability, and flavor -enhancement (2); however, it is different in that it contributes virtually no calories to a meal. How is this possible? Dige stible triglycerides consist of three fatty acids joined by ester linkages to an alcohol known as glycerol. Esters of alcohols with more than three fatty acids become more and more difficult to digest and absorb. Six fatty acids joined in ester linkages to a central alcohol moiety are enough to render the molecule insusceptible to hydrolytic cleavage by pancreatic lipases.(1,2) Since lipids (even typical triglycerides) cannot be absorbed across the intestinal mucosa without first being broken down, olestra moves through the intestine without being absorbed and exits the gastrointestinal (GI) tract intact. What is the difference between olestra and existing fat substitutes available to the food industry? Although fat -free foods have been around for some time, olestra is the first substitute that does not degrade when cooked.(2) Olestra potato chips, for example, will be fried rather than baked, thus offering the oozing, greasy, can't -eat -just -one appeal of the original minus the fat. This sounds like a dream come true for an obese America and the doctors who cannot convince their patients to reduce their excessive fat intake levels even when the risks include coronary heart disease and cancer.(3) Olestra does, however, have its opponents, mainly in the health care and public health fields, and the fact that the U.S. -

Taste Bud Hackers Flavours and Nutrition Research
OUTLOOK TASTE multinational PepsiCo based in Purchase, New York,. “So when you start to talk about changing fat in a food, you’re going to be changing all of CHRIS LOSS those things.” Which means, she says, it’s very dif- ficult to create a product that gets it all right. The food industry is hoping that an updated under- standing of taste and its underlying biology will yield flavoursome formulations that are better for us than the products on the shelves today. FISHING FOR FLAVOURS One of the major steps forward in taste sci- ence in recent decades has been the discovery and exploration of distinct taste receptors on the human tongue. Our taste buds have sepa- rate receptors for, at the very least, five basic tastes: sweet, salty, sour, bitter and umami (savoury). Of these, the receptors for sweet, bitter and umami are all members of a family of proteins called G-protein-coupled recep- tors (GPCRs). And because GPCRs are well understood, they provide numerous oppor- tunities for scientists looking for molecules that might trigger them. Enter Chromocell and Senomyx, based in San Diego, California. These companies have adapted high-throughput screening systems developed by the pharmaceutical industry to find potential drug candidates against GPCRs, to identify molecules that interact with taste cells. “The whole idea of using molecular biol- ogy to trick or tweak your taste buds is kind of novel for the food industry,” says Beverly Tepper, a taste researcher at Rutgers University in New Brunswick, New Jersey. The screening systems work by running a slew of molecules past a panel of taste receptors The combined hot and cold sensations of chilli and peppercorn might make a tasty substitute to salt. -

Module 3-4 | Test 1A | 2015
Module 1-6 | Test 3 | 2015 1 Module 1 – 6 PLEASE DO NOT MARK ON THIS COPY. USE YOUR SCANTRONS TO MARK UNSWERS. Test 3 This is a 50-question exam. Each question is worth two points. Based upon the information provided in the following case study, and the knowledge, skills, and competencies you have acquired during the semester, please solve each question and choose the best answer. You will have two hours to complete the exam. Case Study Information for Bobby and Jean Bobby and Jean are married, both age 22, and very busy college students. They both work and go to school full-time. They believe that there is just not enough time in their weekly schedules for fitness activities or home cooking. Their exercise amounts to cleaning the apartment once a week for two hours. Recently, they both answered the Consumer Education Health Survey. Bobby shared his results with a friend who is a human performance management major. His friend did a separate voluntary health assessment on Bobby. These results are below. Jean is pregnant and weighs 150 pounds. Jean’s current favorite food is canned chili with hot beans (a separate food label for this food is provided). One day Jean snacked on some canned chili with hot beans. After about eight hours, she began to experience nausea and diarrhea. While she was at home sick, she read an article in Consumer Education magazine. The article was written by the director of the local gym. He wrote the article on how to counter the ill effects of stress using diet and exercise. -

Investigation of Commodity Food Standards and Food Additives in Asia”(Ⅲ)
FY2011 Financial Supports for Promoting the “Sixth Industry” in Agriculture, Forestry and Fisheries and Rural Areas Creation and Promotion of the “Sixth Industry” for Pioneering the Future Overseas Business Support Project for Japanese Food Industry in East Asia “Investigation of Commodity Food Standards and Food Additives in Asia”(Ⅲ) Reported by : Hiroaki Hamano, International Life Sciences Institute (ILSI Japan) Investigation Ryoichi Akahoshi (Yakult Honsha Co., Ltd.) Collaborator : Yumi Asada (Unilever Japan Co.) Hiroshi Iwamoto (Morinaga Milk Industry Co., Ltd.) Youichiro Umeki (Danisco Japan Co.) Toshihisa Ohta (Yakult Honsha Co., Ltd.) Hiromi Ohta (Suntory Wellness Ltd.) Yoko Ogiwara (Ajinomoto Co., Inc. ASEAN Regional HQs) Satoru Kasai (Nihon Kraft Foods Limited) Kiyohisa Kaneko (Coca-Cola (Japan) Co., Ltd.) Kaori Kusano (Kirin Group Office Company, Ltd.) Yoshiharu Kuma (Kuma Consulting Engineer Office) Masanori Kohmura (Ajinomoto Co., Inc.) Yukio Suzuki (Schiff's Japan) Fumiko Sekiya (Takasago International Co.) Tomoko Takahashi (Nestle Japan Ltd.) Hisahiro Tatewaki (Kirin Holdings Company, Ltd.) Hidekazu Hosono (Suntory Business Expert Ltd.) Kensuke Watanabe (Suntory Business Expert Ltd.) Ryuji Yamaguchi (ILSI Japan) Hisami Shinohara (ILSI Japan) Shuji Iwata (ILSI Japan) Kazuo Sueki (ILSI Japan) ILSI Korea ILSI Focal Point in China ILSI Southeast Asia Region 1. Purpose of the Investigation In order to strengthen management practices and international competitiveness of Japanese food industry that is facing quantitative saturation and maturity in domestic market, it is necessary to address developing business in East Asian - 1 - regions where attractive market is forming due to increasing population and dynamically growing economy. In the past, Japanese food industry has been reluctant to develop new business in East Asia due to lack of information and understanding on food standards, methods of analysis, and conditions for use of food additives in the countries. -

Codex Alimentarius Commission FOOD and AGRICULTURE WORLD HEALTH ORGANIZATION ORGANIZATION of the UNITED NATIONS
codex alimentarius commission FOOD AND AGRICULTURE WORLD HEALTH ORGANIZATION ORGANIZATION OF THE UNITED NATIONS JOINT OFFICE: Via delle Terme di Caracalla 00100 ROME: Tel. 5797 Cables Foodagri ALINORM 78/12 JOINT FAO/WHO FOOD STANDARDS PROGRAMME CODEX ALIMENTARIUS COMMISSION Twelfth Session, April 1978 REPORT OF THE ELEVENTH SESSION OF THE CODEX COMMITTEE ON FOOD ADDITIVES The Hague, 31 May - 6 June 1977 TABLE OF CONTENTS Page Introduction, Appointment of Rapporteurs, Adoption of the Agenda 1 Appointment of Working Groups 1 Matters of Interest to the Committee 2 Consideration of the Report of the ad hoc Working Group on Flavours 5 Report of the ad hoc Working Group on Food Additive Intake 6 Endorsement of Food Additives in Codex Commodity Standards 7 Endorsement of Maximum Levels for Contaminants in Codex Commodity 11 Standards Consideration of Hydrolyzed Protein 12 List C of Food Additives 13 Establishment of Revised Codex List of Food Additives 13 Lists A and C 14 Advisory List of Additives in Soft Drinks 14 Consideration of Processing Aids 15 Consideration of the Report of the Working Group on Specifications 16 Specifications for Food Grade Salt 17 Revised Proposed Draft General Standard for the Labelling of Food Additives 17 when Sold as such Consideration of the Food Irradiation Process 18 Priority List for Food Additives 21 Note Concerning the Various ad hoc Working Groups 21 Future work 21 Other Business 21 Time and Place of Next Session 21 Closure of the Session 22 APENDICES Appendix I - List of Participants 22 Appendix II - Report -

E Number from Wikipedia, the Free Encyclopedia
E number From Wikipedia, the free encyclopedia E numbers are codes for substances which can be used as food additives for use within the European Union[1] and Switzerland (the "E" stands for "Europe").[2] They are commonly found on food labels throughout the European Union.[3] Safety assessment and approval are the responsibility of the European Food Safety Authority.[4] Having a single unified list for food additives was first agreed upon in 1962 with colours. In 1964, the directives for preservatives were added, 1970 for antioxidants and 1974 for the emulsifiers, stabilisers, thickeners and gelling agents.[5] Contents A solution of E101 riboflavin (also 1 Numbering scheme known as Vitamin B2) 2 Colloquial use 3 Classification by numeric range 4 Full list 4.1 E100–E199 (colours) 4.2 E200–E299 (preservatives) 4.3 E300–E399 (antioxidants, acidity regulators) 4.4 E400–E499 (thickeners, stabilizers, emulsifiers) 4.5 E500–E599 (acidity regulators, anti-caking Crystals of E621 Monosodium glutamate, a flavour enhancer agents) 4.6 E600–E699 (flavour enhancers) 4.7 E700–E799 (antibiotics) 4.8 E900–E999 (glazing agents and sweeteners) 4.9 E1000–E1599 (additional chemicals) 5 See also 6 Notes 7 External links Numbering scheme The numbering scheme follows that of the International Numbering System (INS) as determined by the Codex Alimentarius committee,[6] though only a subset of the INS additives are approved for use in the European Union as food additives. E numbers are also encountered on food labelling in other jurisdictions, including the Cooperation Council for the Arab States of the Gulf, Australia, New Zealand[7] and Israel. -

(1999) 719 Final WHITE PAPER on FOOD SAFETY
COMMISSION OF THE EUROPEAN COMMUNITIES Brussels, 12 January 2000 COM (1999) 719 final WHITE PAPER ON FOOD SAFETY TABLE OF CONTENTS EXECUTIVE SUMMARY.....................................................................................................3 CHAPTER 1: INTRODUCTION ...........................................................................................6 CHAPTER 2: PRINCIPLES OF FOOD SAFETY ..................................................................8 CHAPTER 3: ESSENTIAL ELEMENTS OF FOOD SAFETY POLICY: INFORMATION GATHERING AND ANALYSIS – SCIENTIFIC ADVICE .................................................10 CHAPTER 4: TOWARDS ESTABLISHING A EUROPEAN FOOD AUTHORITY...........14 CHAPTER 5: REGULATORY ASPECTS...........................................................................22 CHAPTER 6: CONTROLS ..................................................................................................29 CHAPTER 7: CONSUMER INFORMATION .....................................................................31 CHAPTER 8: INTERNATIONAL DIMENSION.................................................................34 CHAPTER 9: CONCLUSIONS ...........................................................................................36 ANNEX ...............................................................................................................................37 EXECUTIVE SUMMARY Assuring that the EU has the highest standards of food safety is a key policy priority for the Commission. This White Paper reflects this priority. A radical