Food Additives
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Harmful Effects of Food Preservatives on Human Health Shazia Khanum Mirza1, U.K
Journal of Medicinal Chemistry and Drug Discovery ISSN: 2347-9027 International peer reviewed Journal Special Issue Analytical Chemistry Teacher and Researchers Association National Convention/Seminar Issue 02, Vol. 02, pp. 610-616, 8 January 2017 Available online at www.jmcdd.org To Study The Harmful Effects Of Food Preservatives On Human Health Shazia Khanum Mirza1, U.K. Asema2 And Sayyad Sultan Kasim3. 1 -Research student , Dept of chemistry, Maulana Azad PG & Research centre, Aurangabad. 2-3 -Assist prof. Dept of chemistry,Maulana Azad college Arts sci & com.Aurangabad. ABSTRACT Food chemistry is the study of chemical processes and interactions of all biological and non- biological components. Food additives are chemicals added to foods to keep them fresh or to enhance their color, flavor or texture. They may include food colorings, flavor enhancers or a range of preservatives .The chemical added to a particular food for a particular reason during processing or storage which could affect the characteristics of the food, or become part of the food Preservatives are additives that inhibit the growth of bacteria, yeasts, and molds in foods. Additives and preservatives are used to maintain product consistency and quality, improve or maintain nutritional value, maintain palatability and wholesomeness, provide leavening(yeast), control pH, enhance flavour, or provide colour Some additives have been used for centuries; for example, preserving food by pickling (with vinegar), salting, as with bacon, preserving sweets or using sulfur dioxide as in some wines. Some preservatives are known to be harmful to the human body. Some are classified as carcinogens or cancer causing agents. Keywords : Food , Food additives, colour, flavour , texture, preservatives. -

Chapter 8: Food Additives
8 Food Additives Tanya Louise Ditschun and Carl K. Winter CONTENTS Introduction Food Additive Functionality Food Additive Regulations Generally Recognized as Safe (GRAS) The Delaney Clause Unintentional Additives Assessment of Food Safety` Specific Food Additives Under Scrutiny Saccharin Aspartame Hydrolysis Products of Aspartame Aspartic Acid Phenylalanine Methanol Diketopiperazine Marketing of Aspartame Erythrosine (FD & C Red #3) Olestra Anectodal Reports of Health Effects Due to Olestra Effects of Olestra on Nutrient Absorption Vitamin A Vitamin E Vitamin D Vitamin K Triglycerides Dietary Phytochemicals References © 2000 by CRC Press LLC Introduction Food additives have been used for centuries in food processing practices such as smoking and salting meat. Prior to the advent of refrigeration, food grown in the summer had to be preserved for the winter; salt, sugar, and vinegar were commonly used preservatives. The pursuits of explorers such as Marco Polo were often for food additives. Additives serve many roles and common uses include maintaining product consistency and pal- atability, providing leavening or control pH, enhancing flavor, and impart- ing color. A food additive can be defined in many ways. The Codex Alimentarius Commission, which develops international regulatory guidelines for food additives, provides the following definition of a food additive: Any substance not normally consumed as a food by itself, and not normal- ly used as a typical ingredient of the food, whether or not it has nutritive value, the intentional addition of which to food for a technological (includ- ing organoleptic) purpose in the manufacture, processing, preparation, treatment, packing, packaging, transport or holding of such food results, or may reasonably be expected to result, directly or indirectly, in it or its by-products becoming a component of or otherwise affecting the charac- teristics of such food. -

Supporting Document 1
Supporting document 1 Risk and Technical Assessment Report – Application A1103 Citric & Lactic Acids as Food Additives in Beer and related products Executive Summary FSANZ received an Application from DB Breweries Limited seeking to amend Standard 1.3.1 – Food Additives of the Australia New Zealand Food Standards Code (the Code) to permit the addition of citric and lactic acids as food additives in beer. Within Standard 1.3.1, this permission would apply to the food category 14.2.1 - Beer and related products. Citric and lactic acids are currently permitted as food additives in a large range of foods at levels consistent with Good Manufacturing Practice (GMP). For the proposed use of citric and lactic acids in beer and related products, this Application also requests GMP permission. The food technology assessment concluded that certain types of beers exhibit improved flavour profiles due to pH reduction achieved by the addition of citric and lactic acids. Maximum levels of addition are expected to be approximately 3 g per litre of beer (total of citric acid plus lactic acid). Citric acid and lactic acid are substances of very low toxicity. Citric acid occurs in many foods, with levels of approximately 10 and 50 g/L in orange juice and lemon juice, respectively. Lactic acid levels are highest in foods produced by fermentation, with levels of approximately 10 g/kg reported for cheese and yogurt. The Joint FAO/WHO Expert Committee on Food Additives (JECFA) concluded that establishment of an Acceptable Daily Intake (ADI) expressed in numerical terms was unnecessary for either substance. -
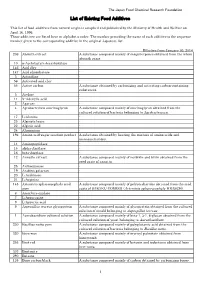
List of Existing Food Additives
The Japan Food Chemical Research Foundation List of Existing Food Additives This list of food additives from natural origin is complied and published by the Ministry of Health and Welfare on April 16, 1996. These additives are listed here in alphabetic order. The number preceding the name of each additive is the sequence number given to the corresponding additive in the original Japanese list. Effective from January 30, 2014 236 Absinth extract A substance composed mainly of sesquiterpenes obtained from the whole absinth grass. 10 α-Acetolactate decarboxylase - 146 Acid clay - 147 Acid phosphatase - 3 Actinidine - 56 Activated acid clay - 55 Active carbon A substance obtained by carbonizing and activating carbon-containing substances. 5 Acylase - 11 5'-Adenylic acid - 2 Agarase - 4 Agrobacterium succinoglycan A substance composed mainly of succinoglycan obtained from the cultured solution of bacteria belonging to Agrobacteriurn. 17 L-Alanine - 23 Alginate lyase - 22 Alginic acid - 24 Aluminium - 196 Amino acid-sugar reaction product A substance obtained by heating the mixture of amino acids and monosaccharides. 14 Aminopeptidase - 15 alpha-Amylase - 16 beta-Amylase - 12 Annatto extract A substance composed mainly of norbixin and bixin obtained from the seed coats of annatto. 25 Anthocyanase - 19 Arabino galactan - 20 L-Arabinose - 21 L-Arginine - 145 Artemisia sphaerocephala seed A substance composed mainly of polysaccharides obtained from the seed gum coats of SABAKU-YOMOGI (Artemisia sphaerocephala KRASCH). 6 Ascorbate oxidase - 7 L-Asparagine - 8 L-Aspartic acid - 9 Aspergillus terreus glycoprotein A substance composed mainly of glycoprotein obtained from the cultured solution of mould belonging to Aspergillus terreus . 1 Aureobasidium cultured solution A substance composed mainly of beta-1, 3-1, 6-glucan obtained from the cultured solution of yeast belonging to Aureobasidium . -

Electronic Nicotine Delivery Device (ENDS)"
Qeios, CC-BY 4.0 · Review, March 4, 2020 Review of "Electronic Nicotine Delivery Device (ENDS)" Clive Bates Overall. T he definition provided is helpful but there are a few remaining ambiguities, most notably with respect to the inclusion or exclsuion of heated tobacco products within this definition. I think the set of definitions in this field should be looked at together for coherence, and speculate whether they could be built from a set of elemental variables. Device? Strictly, ENDS are not devices, but device-liquid combinations. It is possible to put a non-nicotine liquid in a refillable vaping device that is also designed for nicotine (i.e a flavoured e-liquid with no nicotine). So a device only becomes an ENDS when paired with liquid or class of liquids that contain nicotine. T he same hardware is not an ENDS if not using a nicotine liquid. Not all devices are hand-held: there are electronic hookah pipes using nicotine liquids, for example, that stand on a table or the floor. H umectant? T he function of the propylene glycol or glycerol in the e-liquid is not primarily as a humectant but as: (1) a diluent (to create a chosen nicotine concentration) and, (2) an excipient (a inert carrier for the active ingredients - flavours and nicotine). While these agents can also be humectants, that is not their primary function in ENDS. Clarity over excipient names. T here is great confusion about the names of excipients and an opportunity for clarity here: "...one or more excipients, which may include propylene glycol (PG), glycerol or other excipients. -

Propylene Glycol
PROPYLENEGLYCOL ____________________Name ____________________________________________DateDate alsocalled…methylethylglycol , propane-1,2-diol , 1,2-propanediol ,2-hydroxypropanol , 1,2-dihydroxypropane , isopropyleneglycol ,and E1520 . Whatisit? Propyleneglycolisusedasasofteningagent,solvent,moisturizer,preservativeor vehicleinmanypersonalproducts,medications,andindustry. Wheremightitbefound? Heattransferfluid Householdcleaningproducts Moisturizinglotion,cream Hydraulicpressfluid Make-up(foundation,concealer, Industrialsolvents lipstick,lipliner,lipbalm, Insecttrapcontents gloss,mascara,eyeliner) Paint,enamel,stain,deckcoat,varnish Hairproducts(shampoo,gel, Paintballingredient conditioner,color,minoxidil) Petshampoo,spray,deodorizer Soap,cleanser,bodywash Photographicchemical Bubblebath,showergel Pitfalltrapforgroundbeetles Handsanitizer,handcleaner Plasticizer,polyesterandalkydresins Moisttowelettes,babywipes Polyurethanecushions Toothpaste,toothwhiteners Printingfountaininksolution,rollerwash Mouthwash,coldsoreremedy Tiresealant Shavingcream,aftershavegel Tobaccohumectant,cigarhumidor Antiperspirant,deodorant Transcutaneous-nervestimulatorgel Cuticleremover Ultraviolettattooink Salinesolution Wallpaperstripper,drywallprimer Personallubricant Waterproofing,cracksealant Sunscreen,massageoil Treatmentforathletesfoot,itch, PGisinmanyprescriptions : acne,yeast,earache Most cortisone creams,ointments,lotions,gels Clindamycingel,sol’nKeralytGel OtherPossibleExposures: Dovonexsolution Ketoconazolecr,foam Aircraftde-icingfluid Efudexcream,sol’nMetronidazolegel -

Clean Label Alternatives in Meat Products
foods Review Clean Label Alternatives in Meat Products Gonzalo Delgado-Pando 1 , Sotirios I. Ekonomou 2 , Alexandros C. Stratakos 2 and Tatiana Pintado 1,* 1 Institute of Food Science, Technology and Nutrition (CSIC), José Antonio Novais 10, 28040 Madrid, Spain; [email protected] 2 Centre for Research in Biosciences, Coldharbour Lane, Faculty of Health and Applied Sciences, University of the West of England, Bristol BS16 1QY, UK; [email protected] (S.I.E.); [email protected] (A.C.S.) * Correspondence: [email protected] Abstract: Food authorities have not yet provided a definition for the term “clean label”. However, food producers and consumers frequently use this terminology for food products with few and recognisable ingredients. The meat industry faces important challenges in the development of clean-label meat products, as these contain an important number of functional additives. Nitrites are an essential additive that acts as an antimicrobial and antioxidant in several meat products, making it difficult to find a clean-label alternative with all functionalities. Another important additive not complying with the clean-label requirements are phosphates. Phosphates are essential for the correct development of texture and sensory properties in several meat products. In this review, we address the potential clean-label alternatives to the most common additives in meat products, including antimicrobials, antioxidants, texturisers and colours. Some novel technologies applied for the development of clean label meat products are also covered. Keywords: clean label; meat products; nitrites alternatives; phosphates alternatives Citation: Delgado-Pando, G.; Ekonomou, S.I.; Stratakos, A.C.; Pintado, T. -

Evaluation of Honey and Rice Syrup As Replacements for Sorbitol in the Production of Restructured Duck Jerky
271 Open Access Asian Australas. J. Anim. Sci. Vol. 29, No. 2 : 271-279 February 2016 http://dx.doi.org/10.5713/ajas.15.0431 www.ajas.info pISSN 1011-2367 eISSN 1976-5517 Evaluation of Honey and Rice Syrup as Replacements for Sorbitol in the Production of Restructured Duck Jerky Endy Triyannanto and Keun Taik Lee* Department of Food Processing and Distribution, Gangneung-Wonju National University, Gangneung 210-702, Korea ABSTRACT: The aim of this study was to evaluate the potential of natural humectants such as honey and rice syrup to replace sorbitol in the production of restructured duck jerky. Each humectant was mixed at 3%, 6%, and 10% (wt/wt) concentrations with the marinating solution. The values of water activity and the moisture-to-protein ratio of all of the samples were maintained below 0.75. Jerky samples treated with honey retained more moisture than those exposed to other treatments. Among all samples, those treated with 10% sorbitol produced the highest processing yield and the lowest shear force values. The highest L* value and the lowest b* value were observed for the sorbitol-treated sample, followed by the rice syrup- and honey-treated samples. Duck jerky samples treated with 10% honey showed the highest scores for the sensory parameters evaluated. The overall acceptability scores of samples treated with rice syrup were comparable with those of samples treated with sorbitol. Microscopic observation of restructured duck jerky samples treated with honey showed stable forms and smaller pores when compared with other treatments. (Key Words: Honey, Rice Syrup, Sorbitol, Restructured Duck Jerky) INTRODUCTION use of tenderloin for manufacturing a duck jerky could be valuable because tenderloin has been treated as a by- Meat jerky is a popular snack that is easily found in product and is cheaper than the other parts such as breasts retail shops worldwide. -

Chemical Basics of Life
© Jones & Bartlett Learning, LLC. NOT FOR SALE OR DISTRIBUTION CHAPTER 2 Chemical Basics of Life OUTLINE KEY TERMS Atoms, Molecules, and Chemical Bonds Acids: Electrolytes that release hydrogen ions in water. Atomic Structure Activation energy: The amount of energy required to start a Molecules reaction. Chemical Bonds Anions: Ions with a negative charge. Types of Chemical Reactions Atomic number: A whole number representing the number Enzymes of positively charged protons in the nucleus of an atom. Acids, Bases, and the pH Scale Atomic weight: The total number of protons and neutrons in Chemical Constituents of Cells the nucleus of an atom. Inorganic Substances Atoms: The smallest complete units of an element, varying in Organic Substances size, weight, and interaction with other atoms. Summary Bases: Electrolytes that release ions that bond with Learning Goals hydrogen ions. Critical Thinking Questions Carbohydrates: Substances (including sugars) that provide Websites much of the energy required by the body’s cells, as well as Review Questions helping to build cell structures. Catalysts: Atoms or molecules that can change the rate of a OBJECTIVES reaction without being consumed during the process. After studying this chapter, readers should be able to: Cations: Ions with a positive charge. 1. Describe the relationships between atoms and Chemistry: The study of the composition of matter and molecules. changes in its composition. 2. Explain chemical bonds. Compounds: Molecules made up of different bonded atoms. 3. Describe how an atomic number is determined. Decomposition: A reaction that occurs when bonds with a 4. List the major groups of inorganic chemicals reactant molecule break, forming simpler atoms, molecules, common in cells. -

Food Preservative Capabilities of Grape (Vitis Vinifera) and Clementine Mandarin (Citrus Reticulata) By-Products Extracts in South Africa
sustainability Article Food Preservative Capabilities of Grape (Vitis vinifera) and Clementine Mandarin (Citrus reticulata) By-products Extracts in South Africa Trust M. Pfukwa 1, Olaniyi A. Fawole 2,3, Marena Manley 1 , Pieter A. Gouws 1, Umezuruike Linus Opara 2,3 and Cletos Mapiye 4,* 1 Department of Food Science, Faculty of AgriSciences, Stellenbosch University, Private Bag X1, Matieland, Stellenbosch 7602, South Africa; [email protected] (T.M.P.); [email protected] (M.M.); [email protected] (P.A.G.) 2 Postharvest Technology Research Laboratory, South African Research Chair in Postharvest Technology, Department of Horticultural Sciences, Faculty of AgriSciences, Stellenbosch University, Private Bag X1, Matieland, Stellenbosch 7602, South Africa; [email protected] (O.A.F.); [email protected] (U.L.O.) 3 Postharvest Technology Research Laboratory, South African Research Chair in Postharvest Technology, Department of Food Science, Faculty of AgriSciences, Stellenbosch University, Private Bag X1, Matieland, Stellenbosch 7602, South Africa 4 Department of Animal Sciences, Faculty of AgriSciences, Stellenbosch University, Private Bag X1, Matieland, Stellenbosch 7602, South Africa * Correspondence: [email protected]; Tel.: +27-21-808-2640 Received: 15 February 2019; Accepted: 19 March 2019; Published: 22 March 2019 Abstract: The drive towards sustainable food systems coupled with increased consumer sophistication have prompted innovation in waste valorization. Grape and citrus processing by-products, abundant in the Mediterranean and tropical regions, respectively, are expanding and are sustainable sources of bioactive phytochemicals that can be used as natural preservatives for foods. Phytochemical composition, antioxidant, and antimicrobial properties of extracts from grape pomace (GPE), seeds (GSE), and clementine mandarin peel and pulp (MPE) grown in South Africa were analyzed. -

The Effect of Preservatives and Flavour Additive on the Production of Oxygen-Free Radicals by Isolated Human Neutrophils
International Journal of Nutrition and Food Sciences 2014; 3(3): 210-215 Published online May 30, 2014 (http://www.sciencepublishinggroup.com/j/ijnfs) doi: 10.11648/j.ijnfs.20140303.23 The effect of preservatives and flavour additive on the production of oxygen-free radicals by isolated human neutrophils E. Al-Shammari 1, R. Bano 1, * , S. Khan 1, I. Shankity 2 1Department of Clinical Nutrition, College of Applied Medical Sciences, University of Hail, Hail, Ksa 2Department of Clinical Laboratory Sciences, College of Applied Medical Sciences, University of Hail, Hail, Ksa Email address: [email protected] (R. Bano) To cite this article: E. Al-Shammari, R. Bano, S. Khan, I. Shankity. The Effect of Preservatives and Flavour Additive on the Production of Oxygen-Free Radicals by Isolated Human Neutrophils. International Journal of Nutrition and Food Sciences. Vol. 3, No. 3, 2014, pp. 210-215. doi: 10.11648/j.ijnfs.20140303.23 Abstract: Introduction: Researchers have recently described a relationship between food additives and immune system responses. A decline in immune response has also been observed in people who are in contact with food additives, such as preservatives and flavours found in different foods. Objectives: The objective of the present study was to investigate the in vitro effect of food additives, such as vanillin, Monosodium L- glutamate, Sodium Benzoate, and potassium nitrate, on the production of oxygen free radicals (OFRs) by isolated human nutrophils. Methods: The study was based on the isolation of neutrophils (polymorphonuclear leukocytes; PMNs) from normal blood to determine the effects of preservatives and flavouring additives on the production of Oxygen Free Radicals by stimulated PMNs. -

Formaldehyde
FORMALDEHYDE ____________________Name ____________________________________________DateDate alsocalled…formalin,formicaldehyde,methanal,methylaldehyde,methyleneoxide, oxomethane,oxymethylene,morbicidacid.Formaldehydeisreleasedbythesecommon preservatives: quaternium-15 (Dowicilor1-(3-chloroallyl)-3,5,7-triaza-1-azonia-adamantane- chloride),DMDMhydantoin (Glydantor1,3-dimethylol-5,5-dimethylhydantoin), diazolidinyl urea (GermallII), imidazolidinylurea (Germall,imidurea), 2-bromo-2-nitropropane-1,3-diol (Bronopol),andinindustry trisnitromethane (TrisNitro). Whatisit? Formaldehydeisadisinfectantandpreservative. Wheremightitbefound? Photographicdeveloper Make-upremover,toner Mildewresistantfinish,preservative Moisturizingcream,lotion Embalmingfluid,tissuefixative Make-up,concealer,powder Waterproofglue,adhesive,rubbercement Blush,bronzer Latexemulsions,inksolutions,etchingmaterial Mascara,eyepencil,shadow Water-basedpaint,paintprimer,paintstripper Shampoo,bodycleanser Wax,polish,woodcleaner,waterprooffinish Hairconditioner,tonic,spray Plywood,masonite,particleboard,MDF Hairstylinggel,mousse Asphaltshingles,flooring,fillingagents Sunscreen,sunlesstanner Smokefromwood,coal,kerosene,charcoal Bodypowder,talcumpowder Naturalgascombustion,cigar&cigarettesmoke Mouthwash Aftershave,antiperspirant Howtoavoidit: Bubblebath,liquidsoap Toeliminateexposuretoformaldehyde,checkthe Femininehygienespray,rinse completeingredientlist ofeachproductyouuse. Nailpolish,cuticleremover Checkprescriptionmedicinesandcreamstoo. Fingernailhardener Lookforanyofthenamesabove.Forproducts