Evaluation of Certain Food Additives
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Regulation of Photosynthesis by a Potassium Transporter in the Diatom Phaeodactylum Tricornutum Claire Seydoux
Regulation of photosynthesis by a potassium transporter in the diatom Phaeodactylum tricornutum Claire Seydoux To cite this version: Claire Seydoux. Regulation of photosynthesis by a potassium transporter in the diatom Phaeo- dactylum tricornutum. Vegetal Biology. Université Grenoble Alpes [2020-..], 2020. English. NNT : 2020GRALV031. tel-03172222 HAL Id: tel-03172222 https://tel.archives-ouvertes.fr/tel-03172222 Submitted on 17 Mar 2021 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. THÈSE Pour obtenir le grade de DOCTEUR DE L’UNIVERSITÉ GRENOBLE ALPES Spécialité : Biologie Végétale Arrêté ministériel : 25 mai 2016 Présentée par Claire SEYDOUX Thèse dirigée par Florence COURTOIS, Maitre de conferences, Université Grenoble Alpes et codirigée par Giovanni FINAZZI, Université Grenoble Alpes préparée au sein du Laboratoire Laboratoire de Physiologie Cellulaire Végétale dans l'École Doctorale Chimie et Sciences du Vivant Régulation de la photosynthèse par un transporteur de potassium chez la diatomée Phaeodactylum tricornutum Regulation of photosynthesis -

View Article
Cronicon OPEN ACCESS EC Microbiology Review Article Spirulina Rising: Microalgae, Phyconutrients, and Oxidative Stress Mark F McCarty1 and Nicholas A Kerna2,3* 1Catalytic Longevity, USA 2SMC-Medical Research, Thailand 3First InterHealth Group, Thailand *Corresponding Author: Nicholas A Kerna, (mailing address) POB47 Phatphong, Suriwongse Road, Bangrak, Bangkok, Thailand 10500. Contact: [email protected] Received: August 22, 2019; Pubished: June 30, 2021 DOI: 10.31080/ecmi.2021.17.01135 Abstract Oxidative stress provokes the development of many common diseases and contributes to the aging process. Also, oxidative stress is a critical factor in common vascular disorders and type 2 diabetes. It plays a role in neurodegenerative disorders, such as Alzheimer’s disease, Parkinson’s disease, amytrophic lateral sclerosis, multiple sclerosis, and cancer. Oxidative stress contributes to the healthy regulation of cell function. However, excessive oxidative stress results in pathological processes. Antioxidant vitamins have a limited from oxidative stress, and inhibits NOX. PhyCB, a component of the microalgae Spirulina, shares a similar structure with biliverdin, influence on oxidative stress as most oxidative stress results from the cellular production of superoxide. Bilirubin protects cells a biosynthetic precursor of bilirubin. Thus, the oral administration of PhyCB, phycocyanin, or whole Spirulina shows promise for preventing and or treating human disorders that have resulted from excessive oxidative stress. Keywords: Microalgae; Oxidative Stress; Phycocyanobilin; Phyconutrients; Singlet Oxygen; Spirulina; Superoxide Abbreviations DHA: Docosahexaenoic Acid; HO-1: Heme Oxygenase-1; O2: Oxygen; PhyCB: Phycocyanobilin Introduction To understand how phyconutrients, found abundantly in microalgae, can provide preventive and therapeutic effects, it is fundamental to understand how oxidative stress triggers or contributes to the development of many common diseases—and how microalgae, such as Spirulina can help inhibit oxidative stress in the human body. -

Diverse Biosynthetic Pathways and Protective Functions Against Environmental Stress of Antioxidants in Microalgae
plants Review Diverse Biosynthetic Pathways and Protective Functions against Environmental Stress of Antioxidants in Microalgae Shun Tamaki 1,* , Keiichi Mochida 1,2,3,4 and Kengo Suzuki 1,5 1 Microalgae Production Control Technology Laboratory, RIKEN Baton Zone Program, Yokohama 230-0045, Japan; [email protected] (K.M.); [email protected] (K.S.) 2 RIKEN Center for Sustainable Resource Science, Yokohama 230-0045, Japan 3 Kihara Institute for Biological Research, Yokohama City University, Yokohama 230-0045, Japan 4 School of Information and Data Sciences, Nagasaki University, Nagasaki 852-8521, Japan 5 euglena Co., Ltd., Tokyo 108-0014, Japan * Correspondence: [email protected]; Tel.: +81-45-503-9576 Abstract: Eukaryotic microalgae have been classified into several biological divisions and have evo- lutionarily acquired diverse morphologies, metabolisms, and life cycles. They are naturally exposed to environmental stresses that cause oxidative damage due to reactive oxygen species accumulation. To cope with environmental stresses, microalgae contain various antioxidants, including carotenoids, ascorbate (AsA), and glutathione (GSH). Carotenoids are hydrophobic pigments required for light harvesting, photoprotection, and phototaxis. AsA constitutes the AsA-GSH cycle together with GSH and is responsible for photooxidative stress defense. GSH contributes not only to ROS scavenging, but also to heavy metal detoxification and thiol-based redox regulation. The evolutionary diversity of microalgae influences the composition and biosynthetic pathways of these antioxidants. For example, α-carotene and its derivatives are specific to Chlorophyta, whereas diadinoxanthin and fucoxanthin are found in Heterokontophyta, Haptophyta, and Dinophyta. It has been suggested that Citation: Tamaki, S.; Mochida, K.; Suzuki, K. Diverse Biosynthetic AsA is biosynthesized via the plant pathway in Chlorophyta and Rhodophyta and via the Euglena Pathways and Protective Functions pathway in Euglenophyta, Heterokontophyta, and Haptophyta. -

Myoglobin with Modified Tetrapyrrole Chromophores: Binding Specificity and Photochemistry ⁎ Stephanie Pröll A, Brigitte Wilhelm A, Bruno Robert B, Hugo Scheer A
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Elsevier - Publisher Connector Biochimica et Biophysica Acta 1757 (2006) 750–763 www.elsevier.com/locate/bbabio Myoglobin with modified tetrapyrrole chromophores: Binding specificity and photochemistry ⁎ Stephanie Pröll a, Brigitte Wilhelm a, Bruno Robert b, Hugo Scheer a, a Department Biologie I-Botanik, Universität München, Menzingerstr, 67, 80638 München, Germany b Sections de Biophysique des Protéines et des Membranes, DBCM/CEA et URA CNRS 2096, C.E. Saclay, 91191 Gif (Yvette), France Received 2 August 2005; received in revised form 2 March 2006; accepted 28 March 2006 Available online 12 May 2006 Abstract Complexes were prepared of horse heart myoglobin with derivatives of (bacterio)chlorophylls and the linear tetrapyrrole, phycocyanobilin. Structural factors important for binding are (i) the presence of a central metal with open ligation site, which even induces binding of phycocyanobilin, and (ii) the absence of the hydrophobic esterifying alcohol, phytol. Binding is further modulated by the stereochemistry at the isocyclic ring. The binding pocket can act as a reaction chamber: with enolizable substrates, apo-myoglobin acts as a 132-epimerase converting, e.g., Zn-pheophorbide a' (132S) to a (132R). Light-induced reduction and oxidation of the bound pigments are accelerated as compared to solution. Some flexibility of the myoglobin is required for these reactions to occur; a nucleophile is required near the chromophores for photoreduction (Krasnovskii reaction), and oxygen for photooxidation. Oxidation of the bacteriochlorin in the complex and in aqueous solution continues in the dark. © 2006 Elsevier B.V. -

Photosynthetic Pigments in Diatoms
Mar. Drugs 2015, 13, 5847-5881; doi:10.3390/md13095847 OPEN ACCESS marine drugs ISSN 1660-3397 www.mdpi.com/journal/marinedrugs Review Photosynthetic Pigments in Diatoms Paulina Kuczynska 1, Malgorzata Jemiola-Rzeminska 1,2 and Kazimierz Strzalka 1,2,* 1 Faculty of Biochemistry, Biophysics and Biotechnology, Department of Plant Physiology and Biochemistry, Jagiellonian University, Gronostajowa 7, Krakow 30-387, Poland; E-Mails: [email protected] (P.K.); [email protected] (M.J.-R.) 2 Małopolska Centre of Biotechnology, Gronostajowa 7A, Krakow 30-387, Poland * Author to whom correspondence should be addressed; E-Mail: [email protected]; Tel.: +48-126-646-509; Fax: +48-126-646-902. Academic Editor: Véronique Martin-Jézéquel Received: 10 July 2015 / Accepted: 7 September 2015 / Published: 16 September 2015 Abstract: Photosynthetic pigments are bioactive compounds of great importance for the food, cosmetic, and pharmaceutical industries. They are not only responsible for capturing solar energy to carry out photosynthesis, but also play a role in photoprotective processes and display antioxidant activity, all of which contribute to effective biomass and oxygen production. Diatoms are organisms of a distinct pigment composition, substantially different from that present in plants. Apart from light-harvesting pigments such as chlorophyll a, chlorophyll c, and fucoxanthin, there is a group of photoprotective carotenoids which includes β-carotene and the xanthophylls, diatoxanthin, diadinoxanthin, violaxanthin, antheraxanthin, and zeaxanthin, which are engaged in the xanthophyll cycle. Additionally, some intermediate products of biosynthetic pathways have been identified in diatoms as well as unusual pigments, e.g., marennine. Marine algae have become widely recognized as a source of unique bioactive compounds for potential industrial, pharmaceutical, and medical applications. -

Charles A. S. Hall
Charles A. S. Hall 354 Illick Hall SUNY College of Environmental Science and Forestry One Forestry Drive, Syracuse, NY 13210 (315) 470-6870 or 470-6743 (Secretary); 470-6934 (FAX) CURRICULUM VITAE EDUCATION B.A. Colgate University, Hamilton, NY, Biology 1965 (Advisor: Oran Stanley) M.S. Pennsylvania State University, Univ. Park PA, Zoology 1966 (Advisor: William Cooper) Ph.D. University of North Carolina, Chapel Hill NC, Zoology 1970 (Advisor: H. T. Odum) PROFESSIONAL POSITIONS (Post Ph.D.) 1970 - 1974 Research Associate, Staff Scientist II (half time), Department of Biology, Brookhaven National Laboratory, Upton, NY (Director: George Woodwell) 1975 - 1977 Research Scientist II (half-time), The Ecosystems Center, Marine Biological Laboratory, Woods Hole, MA (Director: George Woodwell) 1972 - 1985 Visiting Assistant Professor, Assistant Professor, Section of Ecology and Systematics, Cornell University, Ithaca, NY 1985 - 1987 Research Associate Professor, Biological Station and Department of Zoology, University of Montana, Yellow Bay and Missoula, MT 1987-1992 Associate Professor, SUNY College of Environmental Science and Forestry, Syracuse, NY 1992 - Professor, SUNY College of Environmental Science and Forestry, Syracuse, NY 2001 - ESF Foundation Distinguished Professor, SUNY College of Environmental Science and Forestry, Syracuse, NY PROFESSIONAL INTERESTS AND GOALS Systems Ecology: The application of integrative tools of science, including especially empirical simulation modeling, to the understanding and management of complex systems of nature and of people and nature. My principal focus throughout the diversity of projects represented herein is, and always has been, the examination of how organisms and societies invest energy in resource exploitation, and how such investments change as the quality of resources changes. -

Chapter 8: Food Additives
8 Food Additives Tanya Louise Ditschun and Carl K. Winter CONTENTS Introduction Food Additive Functionality Food Additive Regulations Generally Recognized as Safe (GRAS) The Delaney Clause Unintentional Additives Assessment of Food Safety` Specific Food Additives Under Scrutiny Saccharin Aspartame Hydrolysis Products of Aspartame Aspartic Acid Phenylalanine Methanol Diketopiperazine Marketing of Aspartame Erythrosine (FD & C Red #3) Olestra Anectodal Reports of Health Effects Due to Olestra Effects of Olestra on Nutrient Absorption Vitamin A Vitamin E Vitamin D Vitamin K Triglycerides Dietary Phytochemicals References © 2000 by CRC Press LLC Introduction Food additives have been used for centuries in food processing practices such as smoking and salting meat. Prior to the advent of refrigeration, food grown in the summer had to be preserved for the winter; salt, sugar, and vinegar were commonly used preservatives. The pursuits of explorers such as Marco Polo were often for food additives. Additives serve many roles and common uses include maintaining product consistency and pal- atability, providing leavening or control pH, enhancing flavor, and impart- ing color. A food additive can be defined in many ways. The Codex Alimentarius Commission, which develops international regulatory guidelines for food additives, provides the following definition of a food additive: Any substance not normally consumed as a food by itself, and not normal- ly used as a typical ingredient of the food, whether or not it has nutritive value, the intentional addition of which to food for a technological (includ- ing organoleptic) purpose in the manufacture, processing, preparation, treatment, packing, packaging, transport or holding of such food results, or may reasonably be expected to result, directly or indirectly, in it or its by-products becoming a component of or otherwise affecting the charac- teristics of such food. -

Algal Pigments As Biomarkers Linking Fish and Benthic Organisms with Type E Botulism
Algal pigments in benthic organisms and fish: development of biomarkers to trace food-web relationships Katherine T. Alben, Maxime Bridoux, Monika Sobiechowska Wadsworth Center, New York State Department of Health Dept. Environmental Health Sciences, SUNY-Albany National Water Quality Monitoring Conference May 9, 2006 San Jose, CA U at ALBANY From R. Ruffin, with permission Type E botulism: what are the food-web pathways? loon grebe gull Hypothesis: algal scud pigments goby yellow orange red can be used to trace food-web connections http://www.combat-fishing.com/lakepondbalance.htm#coldwaterlglake http://www.dnr.state.mn.us/exotics/aquaticanimals/roundgoby/index.html http://www.vancouverisland.com/021wildl&cons/wildlife/birds/cw/cw_herringgull.html http://www.admiraltyaudubon.org/ [email protected] U at ALBANY Algal carotenoids found in the food web diatoms & fucoxanthin C42H60O6 55 chrysophytes diatoxanthin C40H54O2 55 cryptophytes alloxanthin C40H52O2 55 chlorophytes lutein C40H56O2 (5) 55 5 cyanobacteria zeaxanthin C40H56O2 55 5 5 cantha- C40H52O2 55 β-crypto- C40H56O 55 echinenone C40H54O 555 euglenophytes neoxanthin C40H56O4 5 dinoflagellates peridinin C39H52O7 NF NF NF NF crustacean astaxanthin C40H52O4 55 5 5 metabolism U at ALBANY Method Development Standards – high resolution separations Quantitation – NIST, reference materials, MDLs Sample prep – microanalytical procedures, SPE, enzymatic hydrolysis Applications – Phytoplankton Gastropods Dreissenids Fish – round gobies, drum, perch, bass Standards - chlorophyll & carotenoids -

Phototrophic Pigment Production with Microalgae
Phototrophic pigment production with microalgae Kim J. M. Mulders Thesis committee Promotor Prof. Dr R.H. Wijffels Professor of Bioprocess Engineering Wageningen University Co-promotors Dr D.E. Martens Assistant professor, Bioprocess Engineering Group Wageningen University Dr P.P. Lamers Assistant professor, Bioprocess Engineering Group Wageningen University Other members Prof. Dr H. van Amerongen, Wageningen University Prof. Dr M.J.E.C. van der Maarel, University of Groningen Prof. Dr C. Vilchez Lobato, University of Huelva, Spain Dr S. Verseck, BASF Personal Care and Nutrition GmbH, Düsseldorf, Germany This research was conducted under the auspices of the Graduate School VLAG (Advanced studies in Food Technology, Agrobiotechnology, Nutrition and Health Sciences). Phototrophic pigment production with microalgae Kim J. M. Mulders Thesis submitted in fulfilment of the requirement for the degree of doctor at Wageningen University by the authority of the Rector Magnificus Prof. Dr M.J. Kropff, in the presence of the Thesis Committee appointed by the Academic Board to be defended in public on Friday 5 December 2014 at 11 p.m. in the Aula. K. J. M. Mulders Phototrophic pigment production with microalgae, 192 pages. PhD thesis, Wageningen University, Wageningen, NL (2014) With propositions, references and summaries in Dutch and English ISBN 978-94-6257-145-7 Abstract Microalgal pigments are regarded as natural alternatives for food colourants. To facilitate optimization of microalgae-based pigment production, this thesis aimed to obtain key insights in the pigment metabolism of phototrophic microalgae, with the main focus on secondary carotenoids. Different microalgal groups each possess their own set of primary pigments. Besides, a selected group of green algae (Chlorophytes) accumulate secondary pigments (secondary carotenoids) when exposed to oversaturating light conditions. -
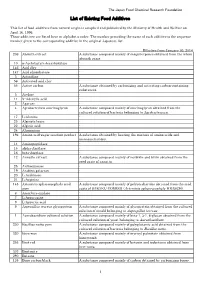
List of Existing Food Additives
The Japan Food Chemical Research Foundation List of Existing Food Additives This list of food additives from natural origin is complied and published by the Ministry of Health and Welfare on April 16, 1996. These additives are listed here in alphabetic order. The number preceding the name of each additive is the sequence number given to the corresponding additive in the original Japanese list. Effective from January 30, 2014 236 Absinth extract A substance composed mainly of sesquiterpenes obtained from the whole absinth grass. 10 α-Acetolactate decarboxylase - 146 Acid clay - 147 Acid phosphatase - 3 Actinidine - 56 Activated acid clay - 55 Active carbon A substance obtained by carbonizing and activating carbon-containing substances. 5 Acylase - 11 5'-Adenylic acid - 2 Agarase - 4 Agrobacterium succinoglycan A substance composed mainly of succinoglycan obtained from the cultured solution of bacteria belonging to Agrobacteriurn. 17 L-Alanine - 23 Alginate lyase - 22 Alginic acid - 24 Aluminium - 196 Amino acid-sugar reaction product A substance obtained by heating the mixture of amino acids and monosaccharides. 14 Aminopeptidase - 15 alpha-Amylase - 16 beta-Amylase - 12 Annatto extract A substance composed mainly of norbixin and bixin obtained from the seed coats of annatto. 25 Anthocyanase - 19 Arabino galactan - 20 L-Arabinose - 21 L-Arginine - 145 Artemisia sphaerocephala seed A substance composed mainly of polysaccharides obtained from the seed gum coats of SABAKU-YOMOGI (Artemisia sphaerocephala KRASCH). 6 Ascorbate oxidase - 7 L-Asparagine - 8 L-Aspartic acid - 9 Aspergillus terreus glycoprotein A substance composed mainly of glycoprotein obtained from the cultured solution of mould belonging to Aspergillus terreus . 1 Aureobasidium cultured solution A substance composed mainly of beta-1, 3-1, 6-glucan obtained from the cultured solution of yeast belonging to Aureobasidium . -

Scalable Production of Biliverdin Ixα by Escherichia Coli Dong Chen1, Jason D Brown1, Yukie Kawasaki2, Jerry Bommer3 and Jon Y Takemoto1,2*
Chen et al. BMC Biotechnology 2012, 12:89 http://www.biomedcentral.com/1472-6750/12/89 RESEARCH ARTICLE Open Access Scalable production of biliverdin IXα by Escherichia coli Dong Chen1, Jason D Brown1, Yukie Kawasaki2, Jerry Bommer3 and Jon Y Takemoto1,2* Abstract Background: Biliverdin IXα is produced when heme undergoes reductive ring cleavage at the α-methene bridge catalyzed by heme oxygenase. It is subsequently reduced by biliverdin reductase to bilirubin IXα which is a potent endogenous antioxidant. Biliverdin IXα, through interaction with biliverdin reductase, also initiates signaling pathways leading to anti-inflammatory responses and suppression of cellular pro-inflammatory events. The use of biliverdin IXα as a cytoprotective therapeutic has been suggested, but its clinical development and use is currently limited by insufficient quantity, uncertain purity, and derivation from mammalian materials. To address these limitations, methods to produce, recover and purify biliverdin IXα from bacterial cultures of Escherichia coli were investigated and developed. Results: Recombinant E. coli strains BL21(HO1) and BL21(mHO1) expressing cyanobacterial heme oxygenase gene ho1 and a sequence modified version (mho1) optimized for E. coli expression, respectively, were constructed and shown to produce biliverdin IXα in batch and fed-batch bioreactor cultures. Strain BL21(mHO1) produced roughly twice the amount of biliverdin IXα than did strain BL21(HO1). Lactose either alone or in combination with glycerol supported consistent biliverdin IXα production by strain BL21(mHO1) (up to an average of 23. 5mg L-1 culture) in fed-batch mode and production by strain BL21 (HO1) in batch-mode was scalable to 100L bioreactor culture volumes. -

Low Acyl Gellan Gum for Inclusion on the National List of Substances Allowed in Organic Production and Handling (7 CFR 205.605 (B)
Petition for Evaluation of Low Acyl Gellan Gum for Inclusion on the National List of Substances Allowed in Organic Production and Handling (7 CFR 205.605 (b) Submitted by: CP Kelco U.S., Inc. 3100 Cumberland Blvd., Suite 600 Atlanta, GA 30339 Date: 08 August 2019 CP Kelco U.S., Inc. 08 August 2019 National Organic List Petiion Low Acyl Gellan Gum Table of Contents Item A.1 — Section of National List ........................................................................................................... 4 Item A.2 — OFPA Category - Crop and Livestock Materials .................................................................... 4 Item A.3 — Inert Ingredients ....................................................................................................................... 4 1. Substance Name ................................................................................................................................... 5 2. Petitioner and Manufacturer Information ............................................................................................. 5 2.1. Corporate Headquarters ................................................................................................................5 2.2. Manufacturing/Processing Facility ...............................................................................................5 2.3. Contact for USDA Correspondence .............................................................................................5 3. Intended or Current Use .......................................................................................................................5