Food Additive Requirements Morocco
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

So2 and Wine: a Review
OIV COLLECTIVE EXPERTISE DOCUMENT SO2 AND WINE: A REVIEW SO2 AND WINE: A REVIEW 1 MARCH 2021 OIV COLLECTIVE EXPERTISE DOCUMENT SO2 AND WINE: A REVIEW WARNING This document has not been submitted to the step procedure for examining resolutions and cannot in any way be treated as an OIV resolution. Only resolutions adopted by the Member States of the OIV have an official character. This document has been drafted in the framework of Expert Group “Food safety” and revised by other OIV Commissions. This document, drafted and developed on the initiative of the OIV, is a collective expert report. © OIV publications, 1st Edition: March 2021 (Paris, France) ISBN 978-2-85038-022-8 OIV - International Organisation of Vine and Wine 35, rue de Monceau F-75008 Paris - France www.oiv.int 2 MARCH 2021 OIV COLLECTIVE EXPERTISE DOCUMENT SO2 AND WINE: A REVIEW SCOPE The group of experts « Food safety » of the OIV has worked extensively on the safety assessment of different compounds found in vitivinicultural products. This document aims to gather more specific information on SO2. This document has been prepared taking into consideration the information provided during the different sessions of the group of experts “Food safety” and information provided by Member States. Finally, this document, drafted and developed on the initiative of the OIV, is a collective expert report. This review is based on the help of scientific literature and technical works available until date of publishing. COORDINATOR OIV - International Organisation of Vine and Wine AUTHORS Dr. Creina Stockley (AU) Dr. Angelika Paschke-Kratzin (DE) Pr. -

Sulfite: Here, There, Everywhere
Sulfite: Here, There, Everywhere Max T. Baker, PhD Associate Professor Department of Anesthesia University of Iowa Inadvertent Exposures Combustion of fossil fuels, Air pollutant Large quantities as sulfur dioxide are expelled from volcanos Kilauea on the Big Island Small quantities endogenously formed in mammals from sulfur-containing amino acid metabolism Deliberate Exposures As Preservative- Wine, Beer (dates to Roman times From burning sulfur candles) Fruits and Vegetables (reduce browning, extend shelf-life) Pharmaceuticals1 Reductant - Antioxidant - Antimicrobial What are Sulfites? Oxidized Forms of the Sulfur Atom Sulfur Dioxide, MW = 64, bp = - 10oC (gaseous) Sulfur (IV) - Oxidation state of 4 S = Atomic number 16 – electrons/shell, 2,8,6 Sodium Dioxide Readily Hydrates2 Sulfur Carbon Dioxide Dioxide (irritant) H O H2O 2 Sulfurous Unstable Carbonic low acid species acid pH high pH Bisulfite Bicarbonate anion anion Sulfite Carbonate dianion dianion Forms radical Doesn’t form radical Bisulfite Can Combine with SO2 to form Metabisulfite + excess Bisulfite Metabisulfite (disulfite, pyrosulfite) “Sulfite” usually added to drugs as sodium or potassium salts of: Sulfite, Bisulfite, or Metabisulfite Endogenous to Mammals Small quantities formed from sulfur-containing amino acid metabolism - cysteine, methionine3 + - + H2O + 2H + 2 e Sulfite Sulfate Rapidly detoxified by sulfite oxidase (SOX) to form sulfate – a two electron oxidation, molybdenum dependent Two Confirmed Sulfite Toxicities Neurological abnormalities from genetic sulfite oxidase deficiency3 Allergic reactions from exogenous exposure4 Oral, parenteral, inhalational exposure: dermatitis, urticaria, flushing, hypotension, abdominal pain and diarrhea to life- threatening anaphylactic and asthmatic reactions “The overall prevalence of sulfite sensitivity in the general population is unknown and probably low. Sulfite sensitivity is seen more frequently in asthmatic than in nonasthmatic people." - FDA Prevalence – 3-10% are sulfite sensitive among asthmatic subjects. -

Reregistration Eligibility Decision (RED) for Inorganic Sulfites
Reregistration Eligibility Decision – Inorganic Sulfites May 2007 Reregistration Eligibility Decision Inorganic Sulfites Special Review and Reregistration Division Office of Pesticide Programs U.S. Environmental Protection Agency 1801 South Bell Street Arlington, VA 22202 Introduction The Environmental Protection Agency (EPA) has completed its Reregistration Eligibility Decision (RED) for the inorganic sulfites case, which includes the chemicals sulfur dioxide and sodium metabisulfite. This assessment provides information to support the issuance of a Reregistration Eligibility Decision for inorganic sulfites. EPA’s pesticide reregistration process provides for the review of older pesticides (those initially registered prior to November 1984) under the Federal Insecticide, Fungicide, and Rodenticide Act (FIFRA) to ensure that they meet current scientific and regulatory standards. In this document, EPA presents the results of its review of the potential human health effects of dietary, drinking water and occupational/bystander exposure to inorganic sulfites, as well as its ecological risk findings. Evaluations performed by the World Health Organization (WHO), the International Agency for Research on Cancer (IARC), and the Agency for Toxic Substances and Disease Registry (ATSDR) were relied upon for this assessment, in addition to peer-reviewed evaluations performed by the Cosmetic Ingredient Review (CIR), the Organization for Economic Cooperation and Development-Screening Information Data Set (OECD-SIDS) and from other open literature sources. Based on this assessment, the Agency has determined that products containing sulfur dioxide or sodium metabisulfite are eligible for reregistration provided the necessary label changes are made. As a result of this assessment, one tolerance has been reassessed. I. Use Information The inorganic sulfites reregistration case includes the chemicals sulfur dioxide (CAS No. -

Chapter 8: Food Additives
8 Food Additives Tanya Louise Ditschun and Carl K. Winter CONTENTS Introduction Food Additive Functionality Food Additive Regulations Generally Recognized as Safe (GRAS) The Delaney Clause Unintentional Additives Assessment of Food Safety` Specific Food Additives Under Scrutiny Saccharin Aspartame Hydrolysis Products of Aspartame Aspartic Acid Phenylalanine Methanol Diketopiperazine Marketing of Aspartame Erythrosine (FD & C Red #3) Olestra Anectodal Reports of Health Effects Due to Olestra Effects of Olestra on Nutrient Absorption Vitamin A Vitamin E Vitamin D Vitamin K Triglycerides Dietary Phytochemicals References © 2000 by CRC Press LLC Introduction Food additives have been used for centuries in food processing practices such as smoking and salting meat. Prior to the advent of refrigeration, food grown in the summer had to be preserved for the winter; salt, sugar, and vinegar were commonly used preservatives. The pursuits of explorers such as Marco Polo were often for food additives. Additives serve many roles and common uses include maintaining product consistency and pal- atability, providing leavening or control pH, enhancing flavor, and impart- ing color. A food additive can be defined in many ways. The Codex Alimentarius Commission, which develops international regulatory guidelines for food additives, provides the following definition of a food additive: Any substance not normally consumed as a food by itself, and not normal- ly used as a typical ingredient of the food, whether or not it has nutritive value, the intentional addition of which to food for a technological (includ- ing organoleptic) purpose in the manufacture, processing, preparation, treatment, packing, packaging, transport or holding of such food results, or may reasonably be expected to result, directly or indirectly, in it or its by-products becoming a component of or otherwise affecting the charac- teristics of such food. -
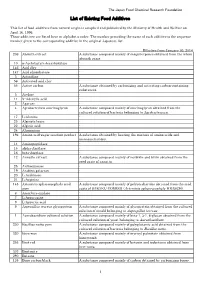
List of Existing Food Additives
The Japan Food Chemical Research Foundation List of Existing Food Additives This list of food additives from natural origin is complied and published by the Ministry of Health and Welfare on April 16, 1996. These additives are listed here in alphabetic order. The number preceding the name of each additive is the sequence number given to the corresponding additive in the original Japanese list. Effective from January 30, 2014 236 Absinth extract A substance composed mainly of sesquiterpenes obtained from the whole absinth grass. 10 α-Acetolactate decarboxylase - 146 Acid clay - 147 Acid phosphatase - 3 Actinidine - 56 Activated acid clay - 55 Active carbon A substance obtained by carbonizing and activating carbon-containing substances. 5 Acylase - 11 5'-Adenylic acid - 2 Agarase - 4 Agrobacterium succinoglycan A substance composed mainly of succinoglycan obtained from the cultured solution of bacteria belonging to Agrobacteriurn. 17 L-Alanine - 23 Alginate lyase - 22 Alginic acid - 24 Aluminium - 196 Amino acid-sugar reaction product A substance obtained by heating the mixture of amino acids and monosaccharides. 14 Aminopeptidase - 15 alpha-Amylase - 16 beta-Amylase - 12 Annatto extract A substance composed mainly of norbixin and bixin obtained from the seed coats of annatto. 25 Anthocyanase - 19 Arabino galactan - 20 L-Arabinose - 21 L-Arginine - 145 Artemisia sphaerocephala seed A substance composed mainly of polysaccharides obtained from the seed gum coats of SABAKU-YOMOGI (Artemisia sphaerocephala KRASCH). 6 Ascorbate oxidase - 7 L-Asparagine - 8 L-Aspartic acid - 9 Aspergillus terreus glycoprotein A substance composed mainly of glycoprotein obtained from the cultured solution of mould belonging to Aspergillus terreus . 1 Aureobasidium cultured solution A substance composed mainly of beta-1, 3-1, 6-glucan obtained from the cultured solution of yeast belonging to Aureobasidium . -

Use of SO2 in High-Ph Wines Sulfur Dioxide Dosage
Purdue extension FS-52-W Commercial Winemaking Production Series Use of SO2 in High-pH Wines Sulfur dioxide dosage By Christian Butzke Wine pH and alcohol destroy the vitamin thiamin, which is essential for the growth of Enology Professor How much free sulfur dioxide (SO2) must a winemaker add or measure to prevent Brettanomyces yeast and certain wine Department of Food Science malolactic fermentation or Brettanomyces bacteria. Only proper concentrations of Purdue University growth if a wine’s pH is 3.95? The answer free SO2 provide additional capacity to bind more products of oxidative aging, [email protected] is between 79 and 112 mg/L, depending on the alcohol content of the wine. The to cleave thiamin, or to kill unwanted SO -sensitive microbes. Brettanomyces requirements for free SO2 concentrations 2 in wine increase exponentially with pH, thrives at higher pH, at temperatures so at pH 4.0 they are 10 times higher greater than 55˚F, in larger ullages, and than at pH 3.0. This does not leave room at residual yeast nutrient levels. Luckily for rule-of-thumb or routine sulfite the thiamin break-up — given proper additions/adjustments. Sulfites added amounts of free bisulfite — occurs faster at a higher pH. Ethanol acts synergistically to wine in the form of either SO2 gas or potassium metabisulfite salt exist and enhances the bacteria-killing effect essentially in two forms: ionized bisulfite of molecular SO2, so high-alcohol wines require less SO protection (see dosage (free SO2) and sulfur dioxide gas 2 charts based on wine alcohol content (molecular SO2). -

Safety Assessment of Sulfites As Used in Cosmetics
Safety Assessment of Sulfites as Used in Cosmetics Status: Re-Review for Panel Consideration Release Date: August 22, 2019 Panel Meeting Date: September 16-17, 2019 The 2019 Cosmetic Ingredient Review Expert Panel members are: Chair, Wilma F. Bergfeld, M.D., F.A.C.P.; Donald V. Belsito, M.D.; Curtis D. Klaassen, Ph.D.; Daniel C. Liebler, Ph.D.; James G. Marks, Jr., M.D., Ronald C. Shank, Ph.D.; Thomas J. Slaga, Ph.D.; and Paul W. Snyder, D.V.M., Ph.D. The CIR Executive Director is Bart Heldreth, Ph.D. This safety assessment was prepared by Wilbur Johnson, Jr., Senior Scientific Analyst © Cosmetic Ingredient Review 1620 L Street, NW, Suite 1200 ♢ Washington, DC 20036-4702 ♢ ph 202.331.0651 ♢ fax 202.331.0088 ♢ [email protected] Distributed for Comment Only -- Do Not Cite or Quote Commitment & Credibility since 1976 Memorandum To: CIR Expert Panel Members and Liaisons From: Wilbur Johnson, Jr. Senior Scientific Analyst Date: August 22, 2019 Subject: Re-Review of the Safety Assessment of Sulfites The CIR Expert Panel first reviewed the safety of Sulfites in 2003. The Panel concluded that Ammonium Bisulfite, Ammonium Sulfite, Potassium Metabisulfite, Potassium Sulfite, Sodium Bisulfite, Sodium Metabisulfite, and Sodium Sulfite are safe as used in cosmetic formulations. The original report is included for your use (identified as sulfit092019orig in the pdf). Minutes from the deliberations of the original review are also included (sulfit092019min_orig). Because it has been at least 15 years since the safety assessment was published, in accordance with CIR Procedures, the Panel should consider whether the safety assessment of Sulfites should be reopened. -

Clean Label Alternatives in Meat Products
foods Review Clean Label Alternatives in Meat Products Gonzalo Delgado-Pando 1 , Sotirios I. Ekonomou 2 , Alexandros C. Stratakos 2 and Tatiana Pintado 1,* 1 Institute of Food Science, Technology and Nutrition (CSIC), José Antonio Novais 10, 28040 Madrid, Spain; [email protected] 2 Centre for Research in Biosciences, Coldharbour Lane, Faculty of Health and Applied Sciences, University of the West of England, Bristol BS16 1QY, UK; [email protected] (S.I.E.); [email protected] (A.C.S.) * Correspondence: [email protected] Abstract: Food authorities have not yet provided a definition for the term “clean label”. However, food producers and consumers frequently use this terminology for food products with few and recognisable ingredients. The meat industry faces important challenges in the development of clean-label meat products, as these contain an important number of functional additives. Nitrites are an essential additive that acts as an antimicrobial and antioxidant in several meat products, making it difficult to find a clean-label alternative with all functionalities. Another important additive not complying with the clean-label requirements are phosphates. Phosphates are essential for the correct development of texture and sensory properties in several meat products. In this review, we address the potential clean-label alternatives to the most common additives in meat products, including antimicrobials, antioxidants, texturisers and colours. Some novel technologies applied for the development of clean label meat products are also covered. Keywords: clean label; meat products; nitrites alternatives; phosphates alternatives Citation: Delgado-Pando, G.; Ekonomou, S.I.; Stratakos, A.C.; Pintado, T. -

Chemical Basics of Life
© Jones & Bartlett Learning, LLC. NOT FOR SALE OR DISTRIBUTION CHAPTER 2 Chemical Basics of Life OUTLINE KEY TERMS Atoms, Molecules, and Chemical Bonds Acids: Electrolytes that release hydrogen ions in water. Atomic Structure Activation energy: The amount of energy required to start a Molecules reaction. Chemical Bonds Anions: Ions with a negative charge. Types of Chemical Reactions Atomic number: A whole number representing the number Enzymes of positively charged protons in the nucleus of an atom. Acids, Bases, and the pH Scale Atomic weight: The total number of protons and neutrons in Chemical Constituents of Cells the nucleus of an atom. Inorganic Substances Atoms: The smallest complete units of an element, varying in Organic Substances size, weight, and interaction with other atoms. Summary Bases: Electrolytes that release ions that bond with Learning Goals hydrogen ions. Critical Thinking Questions Carbohydrates: Substances (including sugars) that provide Websites much of the energy required by the body’s cells, as well as Review Questions helping to build cell structures. Catalysts: Atoms or molecules that can change the rate of a OBJECTIVES reaction without being consumed during the process. After studying this chapter, readers should be able to: Cations: Ions with a positive charge. 1. Describe the relationships between atoms and Chemistry: The study of the composition of matter and molecules. changes in its composition. 2. Explain chemical bonds. Compounds: Molecules made up of different bonded atoms. 3. Describe how an atomic number is determined. Decomposition: A reaction that occurs when bonds with a 4. List the major groups of inorganic chemicals reactant molecule break, forming simpler atoms, molecules, common in cells. -

Investigation of Commodity Food Standards and Food Additives in Asia”(Ⅲ)
FY2011 Financial Supports for Promoting the “Sixth Industry” in Agriculture, Forestry and Fisheries and Rural Areas Creation and Promotion of the “Sixth Industry” for Pioneering the Future Overseas Business Support Project for Japanese Food Industry in East Asia “Investigation of Commodity Food Standards and Food Additives in Asia”(Ⅲ) Reported by : Hiroaki Hamano, International Life Sciences Institute (ILSI Japan) Investigation Ryoichi Akahoshi (Yakult Honsha Co., Ltd.) Collaborator : Yumi Asada (Unilever Japan Co.) Hiroshi Iwamoto (Morinaga Milk Industry Co., Ltd.) Youichiro Umeki (Danisco Japan Co.) Toshihisa Ohta (Yakult Honsha Co., Ltd.) Hiromi Ohta (Suntory Wellness Ltd.) Yoko Ogiwara (Ajinomoto Co., Inc. ASEAN Regional HQs) Satoru Kasai (Nihon Kraft Foods Limited) Kiyohisa Kaneko (Coca-Cola (Japan) Co., Ltd.) Kaori Kusano (Kirin Group Office Company, Ltd.) Yoshiharu Kuma (Kuma Consulting Engineer Office) Masanori Kohmura (Ajinomoto Co., Inc.) Yukio Suzuki (Schiff's Japan) Fumiko Sekiya (Takasago International Co.) Tomoko Takahashi (Nestle Japan Ltd.) Hisahiro Tatewaki (Kirin Holdings Company, Ltd.) Hidekazu Hosono (Suntory Business Expert Ltd.) Kensuke Watanabe (Suntory Business Expert Ltd.) Ryuji Yamaguchi (ILSI Japan) Hisami Shinohara (ILSI Japan) Shuji Iwata (ILSI Japan) Kazuo Sueki (ILSI Japan) ILSI Korea ILSI Focal Point in China ILSI Southeast Asia Region 1. Purpose of the Investigation In order to strengthen management practices and international competitiveness of Japanese food industry that is facing quantitative saturation and maturity in domestic market, it is necessary to address developing business in East Asian - 1 - regions where attractive market is forming due to increasing population and dynamically growing economy. In the past, Japanese food industry has been reluctant to develop new business in East Asia due to lack of information and understanding on food standards, methods of analysis, and conditions for use of food additives in the countries. -

An Integrated Approach to Understand Apicomplexan Metabolism From
Shanmugasundram et al. BMC Bioinformatics 2014, 15(Suppl 3):A3 http://www.biomedcentral.com/1471-2105/15/S3/A3 MEETINGABSTRACT Open Access An integrated approach to understand apicomplexan metabolism from their genomes Achchuthan Shanmugasundram1,2*, Faviel F Gonzalez-Galarza1, Jonathan M Wastling2, Olga Vasieva1, Andrew R Jones1 From Ninth International Society for Computational Biology (ISCB) Student Council Symposium 2013 Berlin, Germany. 19 July 2013 Background Methods The Apicomplexa is a large phylum of intracellular para- We have utilised an approach called ‘metabolic recon- sites that show great diversity and adaptability in the struction’, in which genes are systematically assigned to various ecological niches they occupy. They are the cau- functions within pathways/networks [1-4]. Functional sative agents of human and animal infections including annotation and metabolic reconstruction was carried malaria, toxoplasmosis and theileriosis, which have a out using a semi-automatic approach, integrating geno- huge economic and social impact. A number of apicom- mic information with biochemical evidence from the plexan genomes have been sequenced and are publicly literature. The functions were automatically assigned available. However, the prediction of gene models and using a sequence similarity-based approach and protein annotation of gene functions remains challenging. motif information. Experimental evidence was also Table 1 A survey of the data available for the different apicomplexan genomes in LAMP. The analysis is updated from the survey table published in the previous publication [5] Organism No of metabolic No of unique No of missing No of Total no of No of metabolites No of end pathways enzymesa enzymesb reactionsc metabolitesd from hoste metabolites to host or of unknown fatef T. -

Codex Alimentarius Commission FOOD and AGRICULTURE WORLD HEALTH ORGANIZATION ORGANIZATION of the UNITED NATIONS
codex alimentarius commission FOOD AND AGRICULTURE WORLD HEALTH ORGANIZATION ORGANIZATION OF THE UNITED NATIONS JOINT OFFICE: Via delle Terme di Caracalla 00100 ROME: Tel. 5797 Cables Foodagri ALINORM 78/12 JOINT FAO/WHO FOOD STANDARDS PROGRAMME CODEX ALIMENTARIUS COMMISSION Twelfth Session, April 1978 REPORT OF THE ELEVENTH SESSION OF THE CODEX COMMITTEE ON FOOD ADDITIVES The Hague, 31 May - 6 June 1977 TABLE OF CONTENTS Page Introduction, Appointment of Rapporteurs, Adoption of the Agenda 1 Appointment of Working Groups 1 Matters of Interest to the Committee 2 Consideration of the Report of the ad hoc Working Group on Flavours 5 Report of the ad hoc Working Group on Food Additive Intake 6 Endorsement of Food Additives in Codex Commodity Standards 7 Endorsement of Maximum Levels for Contaminants in Codex Commodity 11 Standards Consideration of Hydrolyzed Protein 12 List C of Food Additives 13 Establishment of Revised Codex List of Food Additives 13 Lists A and C 14 Advisory List of Additives in Soft Drinks 14 Consideration of Processing Aids 15 Consideration of the Report of the Working Group on Specifications 16 Specifications for Food Grade Salt 17 Revised Proposed Draft General Standard for the Labelling of Food Additives 17 when Sold as such Consideration of the Food Irradiation Process 18 Priority List for Food Additives 21 Note Concerning the Various ad hoc Working Groups 21 Future work 21 Other Business 21 Time and Place of Next Session 21 Closure of the Session 22 APENDICES Appendix I - List of Participants 22 Appendix II - Report