Ed 229 624 Author Title Srons Agency Note Pub Type
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Speed and Metabolic Cost of Digesting a Blood Meal Depends on Temperature in a Major Disease Vector Marshall D
© 2016. Published by The Company of Biologists Ltd | Journal of Experimental Biology (2016) 219, 1893-1902 doi:10.1242/jeb.138669 RESEARCH ARTICLE The speed and metabolic cost of digesting a blood meal depends on temperature in a major disease vector Marshall D. McCue1,‡, Leigh Boardman2,*, Susana Clusella-Trullas3, Elsje Kleynhans2 and John S. Terblanche2 ABSTRACT and is believed to occur in all animals (reviewed in Jobling, 1983; The energetics of processing a meal is crucial for understanding McCue, 2006; Wang et al., 2006; Secor, 2009). Surprisingly, – energy budgets of animals in the wild. Given that digestion and its information on SDA among one of the largest taxonomic groups – associated costs may be dependent on environmental conditions, it is insects comes from very few studies (Table 1). Furthermore, the necessary to obtain a better understanding of these costs under SDA of insects remains poorly characterized in terms of the diverse conditions and identify resulting behavioural or physiological standard metrics used to characterize this phenomenon in other trade-offs. This study examines the speed and metabolic costs – in animals (e.g. magnitude, peak time, duration and coefficient). Given ’ cumulative, absolute and relative energetic terms – of processing a insects multiple roles as disease vectors, pests of agriculture, and as bloodmeal for a major zoonotic disease vector, the tsetse fly Glossina model taxa for evolutionary, climate and conservation-related brevipalpis, across a range of ecologically relevant temperatures (25, research, this constitutes a significant limitation for integrating 30 and 35°C). Respirometry showed that flies used less energy mechanistic understanding into population dynamics modelling, digesting meals faster at higher temperatures but that their starvation including population persistence and vulnerability to environmental tolerance was reduced, supporting the prediction that warmer change. -

The Harmful Effects of Food Preservatives on Human Health Shazia Khanum Mirza1, U.K
Journal of Medicinal Chemistry and Drug Discovery ISSN: 2347-9027 International peer reviewed Journal Special Issue Analytical Chemistry Teacher and Researchers Association National Convention/Seminar Issue 02, Vol. 02, pp. 610-616, 8 January 2017 Available online at www.jmcdd.org To Study The Harmful Effects Of Food Preservatives On Human Health Shazia Khanum Mirza1, U.K. Asema2 And Sayyad Sultan Kasim3. 1 -Research student , Dept of chemistry, Maulana Azad PG & Research centre, Aurangabad. 2-3 -Assist prof. Dept of chemistry,Maulana Azad college Arts sci & com.Aurangabad. ABSTRACT Food chemistry is the study of chemical processes and interactions of all biological and non- biological components. Food additives are chemicals added to foods to keep them fresh or to enhance their color, flavor or texture. They may include food colorings, flavor enhancers or a range of preservatives .The chemical added to a particular food for a particular reason during processing or storage which could affect the characteristics of the food, or become part of the food Preservatives are additives that inhibit the growth of bacteria, yeasts, and molds in foods. Additives and preservatives are used to maintain product consistency and quality, improve or maintain nutritional value, maintain palatability and wholesomeness, provide leavening(yeast), control pH, enhance flavour, or provide colour Some additives have been used for centuries; for example, preserving food by pickling (with vinegar), salting, as with bacon, preserving sweets or using sulfur dioxide as in some wines. Some preservatives are known to be harmful to the human body. Some are classified as carcinogens or cancer causing agents. Keywords : Food , Food additives, colour, flavour , texture, preservatives. -

Nutrition and Metabolism
NUTRITION AND METABOLISM Metabolism - the sum of the chemical changes that occur in the cell and involve the breakdown (catabolism) and synthesis (anabolism) of stored energy sources. Basal Metabolic Rate is dened as the rate of energy production by the body measured under a dened set of conditions which is usually at rest (physical and mental), room temperature, 12 hours after a meal. The result is produced as a percentage of a standard value which is derived from studies of normal healthy people. Measurement of the metabolic rate takes place using a method called calorimetry. This may be done directly by measuring the amount of heat produced by the body in an Atwater chamber, the metabolic rate is the amount of heat produced per hour. More commonly the metabolic rate is determined indirectly by putting people on a closed circuit breathing system, with CO2 removed by a soda lime scrubber and the rate of oxygen consumption measured by change in volume. Oxygen consumption is proportional to the metabolic rate because most of the energy in the body is derived from oxidative phosphorylation, which uses a set amount of oxygen to produce a set amount of energy. For every litre of oxygen consumed the body produces (uses) 4.82 kcals of energy. If the oxygen consumption is 250ml/min (15L/hr) then the metabolic rate is 72.3 kcals/hr. This is often further rened by dividing the gure by the body surface area which for a 70kg male is 1.73m2. This gives an average BMR of approximately 40 kcal/m2/hr. -

Hunger Banquet
what is a hunger banquet? 3 setting up 4 preparations 5 hunger banquet Schedule 6 Pre-Banquet Discussion 6 Seating & Meal 9 Facilitated Discussion 11 Response 13 appendix A: Admission Tickets 15 appendix B: Room Signage 16 appendix C: Handouts 20 appendix D: Banquet Team Training 22 appendix E: How to Make Dirty Water 23 appendix F: Character Back Stories 24 appendix G: Serving at the FH Sponsorship Table 27 appendix H: Opportunities with Food for the Hungry 30 Hunger Banquet. © 2011 Food for the Hungry. All rights reserved. No part of this publication may be reproduced, stored in a retrieval system, or transmitted in any form by any means, electronic, mechanical, photocopy, recording, or otherwise, without the prior permission of Food for the Hungry, except as provided by USA copyright law. Possession of this document grants you permission to print, photocopy and implement these materials for your group in their original form. Do not modify the material or teach any of the lessons or activities outside the context presented in this document without written consent from Food for the Hungry. You may request permission for such use by contacting the Global Engagement department of Food for the Hungry at [email protected]. 1224 E Washington St, Phoenix, AZ 85034. (800) 248-6437. www.fh.org. The Hunger Banquet® is a registered service mark of Oxfam-America, Inc. (http://www.oxfamamerica.org) and is used herein by permission of the service mark owner. ©2002 Oxfam-America. Inc. All Rights Reserved. Portions of this work were originally published by Oxfam-America, Inc. -
At the End of the Year, Every Classroom in the School Contributed an Article for a School-Wide Newspaper on Our Inquiries Across the School Year
Taking Action in Tight Times Item Type Article; text Authors Edwards, Amy Citation Edwards, Amy. (2009). Taking Action in Tight Times. WOW Stories. Publisher Worlds of Words: Center for Global Literacies and Literatures (University of Arizona) Journal WOW Stories Rights © The Author(s). Open Access. This article is distributed under the terms of the Creative Commons Attribution-NonCommercial- ShareAlike 4.0 International License (CC BY-NC-SA 4.0). Download date 27/09/2021 18:08:00 Item License https://creativecommons.org/licenses/by-nc-sa/4.0/ Version Final published version Link to Item http://hdl.handle.net/10150/651170 At the end of the year, every classroom in the school contributed an article for a school-wide newspaper on our inquiries across the school year. Lisa Thomas, our project coordinator, worked with students from each classroom, reviewing the year in terms of what the students had been learning and working on, and then asking them to choose one idea they saw as significant to write as a class article. I thought that the students would focus on issues of power and control. I knew that they had strong feelings about the decisions in their lives controlled by friends, parents, and teachers and the decisions where they had control. Instead, the students focused on the project to raise money for an animal and they wrote an article describing the project. The article ended with this statement, “We hope that what we donate will keep creating food for hungry people for a very long time.” References Kempf, S. (2005). Finding solutions to hunger. -

Approved: Tre Specific Dynamic Action of Fat and P
TRE SPECIFIC DYNAMIC ACTION OF CARBOHYD.RATF, " FAT AND P.ROJ!EIN IN FIVE WOMEN by P.:l.tricia Jo ~~IG.n.'lley Thesis submitted to the Graduate faculty of the Virginie. Fblytechnic Institute in candidacy for the degree of MAmR OF SCIENCE in RUMAN NllfRITION AND FOODS APPROVED: "Ms.cy W. Korsliind • June, 1966 Blacksburg, Virginia -2- TABLE OF CONTENTS Page ·LIST OF TABLES • • • • • • • • • • • • • • • • • • • • • • 3 LIST OF FIGURES . " . 4 ACKNOWLEDGEMENT • • . .. 5 Chapter 1. INTRODUCTION . 6 II. REVIEW OF LITERATURE • . 9 Summary of Specific Dynamic Action Theories . 9 Specific Dynamic Action of Carbohydrate . • 10 Specific Dynamic Action of Fat , • . 14 Specific Dynamic Action of Protein . .. 15 III. METHODS AND PROCEDURES . 18 Subjects . 18 Adminilt~ation of Food and Measurements . .. 19 Collection and Analysis of Expired Air • . 20 Calculations . • • . • • • . .- . .. 20 IV. RESULTS AND DISCUSSION • • • • • • . .. 21 Changes in Respiratory Quotient Following the Ingestion of Carbohydrate, Fat and Protein •• . 21 V;irr-;iations in He11t Production After Ingestion of Carbohydrate, Fat and Protein • • • 28 V. SUMMARY •••••• . 37 BIBLIOGRAPHY • . 38 VITA •• . ... 44 APPENDIX . 45 TABLE NUMBlll PAGE 1. Time required, aft•l' the inge•tion of carbohydrate by f tve women, to reach highest re•piratory quotient and highest heat production. • • • • • • • • • • • • • • • • 31 2. Maximum increaee in heat production in. four women after ingestion of fat and time required for _.ximum increase to occur •••••••••••••••••• • • 31 3. Ma;xt... tacreaee in heat production in five women after ingeatioa of protein and time required for maximwll increaee to occur. • • • • • • • • • • • •. • • • • • • • 35 -4- LIST OF FIGURES FIGURE NUMBER PAGE 1~ Changes in reepiratory quotient in five women 22 after ingestion of sucrose. -

Chapter 8: Food Additives
8 Food Additives Tanya Louise Ditschun and Carl K. Winter CONTENTS Introduction Food Additive Functionality Food Additive Regulations Generally Recognized as Safe (GRAS) The Delaney Clause Unintentional Additives Assessment of Food Safety` Specific Food Additives Under Scrutiny Saccharin Aspartame Hydrolysis Products of Aspartame Aspartic Acid Phenylalanine Methanol Diketopiperazine Marketing of Aspartame Erythrosine (FD & C Red #3) Olestra Anectodal Reports of Health Effects Due to Olestra Effects of Olestra on Nutrient Absorption Vitamin A Vitamin E Vitamin D Vitamin K Triglycerides Dietary Phytochemicals References © 2000 by CRC Press LLC Introduction Food additives have been used for centuries in food processing practices such as smoking and salting meat. Prior to the advent of refrigeration, food grown in the summer had to be preserved for the winter; salt, sugar, and vinegar were commonly used preservatives. The pursuits of explorers such as Marco Polo were often for food additives. Additives serve many roles and common uses include maintaining product consistency and pal- atability, providing leavening or control pH, enhancing flavor, and impart- ing color. A food additive can be defined in many ways. The Codex Alimentarius Commission, which develops international regulatory guidelines for food additives, provides the following definition of a food additive: Any substance not normally consumed as a food by itself, and not normal- ly used as a typical ingredient of the food, whether or not it has nutritive value, the intentional addition of which to food for a technological (includ- ing organoleptic) purpose in the manufacture, processing, preparation, treatment, packing, packaging, transport or holding of such food results, or may reasonably be expected to result, directly or indirectly, in it or its by-products becoming a component of or otherwise affecting the charac- teristics of such food. -

Artificial Food Colours and Children Why We Want to Limit and Label Foods Containing the ‘Southampton Six’ Food Colours on the UK Market Post-Brexit
Artificial food colours and children Why we want to limit and label foods containing the ‘Southampton Six’ food colours on the UK market post-Brexit November 2020 FIRST STEPS NUTRITIONArtificial food coloursTRUST and children: page Artificial food colours and children: Why we want to limit and label foods containing the‘Southampton Six’ food colours on the UK market post-Brexit November 2020 Published by First Steps Nutrition Trust. A PDF of this resource is available on the First Steps Nutrition Trust website. www.firststepsnutrition.org The text of this resource, can be reproduced in other materials provided that the materials promote public health and make no profit, and an acknowledgement is made to First Steps Nutrition Trust. This resource is provided for information only and individual advice on diet and health should always be sought from appropriate health professionals. First Steps Nutrition Trust Studio 3.04 The Food Exchange New Covent Garden Market London SW8 5EL Registered charity number: 1146408 First Steps Nutrition Trust is a charity which provides evidence-based and independent information and support for good nutrition from pre-conception to five years of age. For more information, see our website: www.firststepsnutrition.org Acknowledgements This report was written by Rachael Wall and Dr Helen Crawley. We would like to thank Annie Seeley, Sarah Weston, Erik Millstone and Anna Rosier for their help and support with this report. Artificial food colours and children: page 1 Contents Page Executive summary 3 Recommendations -
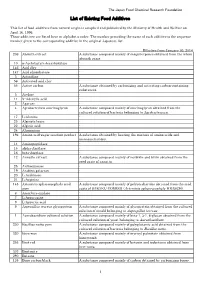
List of Existing Food Additives
The Japan Food Chemical Research Foundation List of Existing Food Additives This list of food additives from natural origin is complied and published by the Ministry of Health and Welfare on April 16, 1996. These additives are listed here in alphabetic order. The number preceding the name of each additive is the sequence number given to the corresponding additive in the original Japanese list. Effective from January 30, 2014 236 Absinth extract A substance composed mainly of sesquiterpenes obtained from the whole absinth grass. 10 α-Acetolactate decarboxylase - 146 Acid clay - 147 Acid phosphatase - 3 Actinidine - 56 Activated acid clay - 55 Active carbon A substance obtained by carbonizing and activating carbon-containing substances. 5 Acylase - 11 5'-Adenylic acid - 2 Agarase - 4 Agrobacterium succinoglycan A substance composed mainly of succinoglycan obtained from the cultured solution of bacteria belonging to Agrobacteriurn. 17 L-Alanine - 23 Alginate lyase - 22 Alginic acid - 24 Aluminium - 196 Amino acid-sugar reaction product A substance obtained by heating the mixture of amino acids and monosaccharides. 14 Aminopeptidase - 15 alpha-Amylase - 16 beta-Amylase - 12 Annatto extract A substance composed mainly of norbixin and bixin obtained from the seed coats of annatto. 25 Anthocyanase - 19 Arabino galactan - 20 L-Arabinose - 21 L-Arginine - 145 Artemisia sphaerocephala seed A substance composed mainly of polysaccharides obtained from the seed gum coats of SABAKU-YOMOGI (Artemisia sphaerocephala KRASCH). 6 Ascorbate oxidase - 7 L-Asparagine - 8 L-Aspartic acid - 9 Aspergillus terreus glycoprotein A substance composed mainly of glycoprotein obtained from the cultured solution of mould belonging to Aspergillus terreus . 1 Aureobasidium cultured solution A substance composed mainly of beta-1, 3-1, 6-glucan obtained from the cultured solution of yeast belonging to Aureobasidium . -

Global Regulations of Food Colors Each Region Has Its Own Definitions of What Constitutes a Color Additive, with Related Use Requirements and Restrictions
Global Regulations of Food Colors Each region has its own definitions of what constitutes a color additive, with related use requirements and restrictions. Sue Ann McAvoy Sensient Colors LLC t is said, we eat with our eyes . Since antiq - food colors was soon recognized as a threat Iuity, humans have used the color of a to public health. Of concern was that some food to discern its quality. Color provides of the substances were known to be poi - a way to judge ripeness, perceive flavor and sonous and were often incorporated to hide assess quality of food. poor quality, add bulk to foods and to pass Ancient civilizations introduced color off imitation foods as real. into their foods. The ancient Egyptians col - On February 1, 1899, the executive com - ored their food yellow with saffron, and the mittee of the National Confectioners’ Asso - ancient Mayans used annatto to color their ciation published an official circular which Sue Ann McAvoy is food orange-red. Wealthy Romans ate bread was “to throw light upon the vexed question global regulatory scien - that had been whitened by adding alum to of what colors may be safely used in confec - tist for Sensient Food the flour. Color could be used to enhance tionery” as “there may at times be a doubt in Colors LLC. She has worked at Sensient the mind of the honest confectioner as to the physical appearance of the product. since 1979. However, if it made the food appear to be which colors, flavors, or ingredients he may of better quality than it was, that was con - safely use and which he may reject.” This list sidered a deceitful practice. -

Specific Dynamic Action
Specific Dynamic Action ➢ The phenomenon of the extra heat production by the body, over and above the calculated caloric value, when a given food is metabolized by the body, is known as specific dynamic action (SDA). ➢ It is also known as calorigenic action or thermogenic action or thermic action (effect) of food. SDA for different foods ➢ For a food containing 25 g of protein, the heat production from the caloric value is 100 Cal (25 x 4 Cal). ➢ When 25 g protein is utilized by the body, 130 Cal of heat is liberated. ➢ The extra 30 Cal is the SDA of protein. ➢ SDA for protein, fat and carbohydrate 32%, 13% & 5%, ➢ Proteins possess the highest SDA while carbohydrates have the lowest. SDA for mixed diet ➢The presence of fats and carbohydrates reduces the SDA of proteins. ➢ Fats are most efficient in reducing SDA of foodstuffs. ➢ For a regularly consumed mixed diet, the SDA is around 10%. Significance of SDA ➢ For the utilization of foods by the body, certain amount of energy is consumed from the body stores. ➢ Expenditure by the body for the utilization of foodstuffs. ➢ It is the highest for proteins (30%) and lowest for carbohydrates (5%) & for mixed diet 10%. ➢ Additional 10% calories should be added to the total energy needs (of the body) towards SDA. ➢ The higher SDA for protein indicates that it is not a good source of energy. Mechanism of SDA ➢ SDA of foods is due to the energy required for digestion, absorption, transport, metabolism and storage of foods in the body. ➢ The SDA of proteins is primarily to meet the energy requirements for deamination, synthesis of urea, biosynthesis of proteins, synthesis of triacylglycerol (from carbon skeleton of amino acids). -

Measurement of Individual and Population Energetics of Marine Mammals
Marine Mammal Ecology and Conservation / 08-Boyd-Ch08 page 165 7:55pm OUP CORRECTED PROOF – Finals, 5/7/2010, SPi 8 Measurement of individual and population energetics of marine mammals Sara J. Iverson, Carol E. Sparling, Terrie M. Williams, Shelley L.C. Lang, and W. Don Bowen 8.1 Introduction The overriding currency of all animal life is energy. Animals have evolved strategies of energy acquisition and use, but these strategies also experience tradeoffs between energy allocated to maintenance, activities, growth, and reproduction and are central to our understanding of life histories and fitness. Thus ‘energetics’— the study of the metabolic requirements, energy use, and output of animals— underpins many areas of physiology, ecology, evolutionary, and population biology, and even ecosystem dynamics. Marine mammals pose many challenges for the study, interpretation, and com- parison of individual and population energetics. For instance, the ability to study captive animals is often restricted to a few of the smaller marine mammal species. Although opportunities in the wild may be greater, there remain serious limits to our abilities to study species in remote locations (e.g. polar bears, Ursus maritimus,in the high arctic), in unstable habitats (e.g. ice-associated pinnipeds in the Bering and Chukchi Seas), or due to endangered status (e.g. monk seals, Monachus spp., southern sea otters, Enhydra lutris nereis, and vaquita, Phocoena sinus). As a group, cetaceans pose further difficulties because of their limited accessibility and large size. Adaptive insulation (blubber) is important in temperature management (Iverson 2009a) and the presence of this comparatively inert tissue can add complexity to the issue of defining the metabolically relevant body mass in marine mammals.