The Speed and Metabolic Cost of Digesting a Blood Meal Depends on Temperature in a Major Disease Vector Marshall D
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Foodservice Toolkit Potatoes Idaho® Idaho® Potatoes
IDAHO POTATO COMMISSION Foodservice Table of Contents Dr. Potato 2 Introduction to Idaho® Potatoes 3 Idaho Soil and Climate 7 Major Idaho® Potato Growing Areas 11 Scientific Distinction 23 Problem Solving 33 Potato Preparation 41 Potato101.com 55 Cost Per Serving 69 The Commission as a Resource 72 Dr. Potato idahopotato.com/dr-potato Have a potato question? Visit idahopotato.com/dr-potato. It's where Dr. Potato has the answer! You may wonder, who is Dr. Potato? He’s Don Odiorne, Vice President Foodservice (not a real doctor—but someone with experience accumulated over many years in foodservice). Don Odiorne joined the Idaho Potato Commission in 1989. During his tenure he has also served on the foodservice boards of United Fresh Fruit & Vegetable, the Produce Marketing Association and was treasurer and then president of IFEC, the International Food Editors Council. For over ten years Don has directed the idahopotato.com website. His interest in technology and education has been instrumental in creating a blog, Dr. Potato, with over 600 posts of tips on potato preparation. He also works with over 100 food bloggers to encourage the use of Idaho® potatoes in their recipes and videos. Awards: The Packer selected Odiorne to receive its prestigious Foodservice Achievement Award; he received the IFEC annual “Betty” award for foodservice publicity; and in the food blogger community he was awarded the Camp Blogaway “Golden Pinecone” for brand excellence as well as the Sunday Suppers Brand partnership award. page 2 | Foodservice Toolkit Potatoes Idaho® Idaho® Potatoes From the best earth on Earth™ Idaho® Potatoes From the best earth on earth™ Until recently, nearly all potatoes grown within the borders of Idaho were one variety—the Russet Burbank. -

Nutrition and Metabolism
NUTRITION AND METABOLISM Metabolism - the sum of the chemical changes that occur in the cell and involve the breakdown (catabolism) and synthesis (anabolism) of stored energy sources. Basal Metabolic Rate is dened as the rate of energy production by the body measured under a dened set of conditions which is usually at rest (physical and mental), room temperature, 12 hours after a meal. The result is produced as a percentage of a standard value which is derived from studies of normal healthy people. Measurement of the metabolic rate takes place using a method called calorimetry. This may be done directly by measuring the amount of heat produced by the body in an Atwater chamber, the metabolic rate is the amount of heat produced per hour. More commonly the metabolic rate is determined indirectly by putting people on a closed circuit breathing system, with CO2 removed by a soda lime scrubber and the rate of oxygen consumption measured by change in volume. Oxygen consumption is proportional to the metabolic rate because most of the energy in the body is derived from oxidative phosphorylation, which uses a set amount of oxygen to produce a set amount of energy. For every litre of oxygen consumed the body produces (uses) 4.82 kcals of energy. If the oxygen consumption is 250ml/min (15L/hr) then the metabolic rate is 72.3 kcals/hr. This is often further rened by dividing the gure by the body surface area which for a 70kg male is 1.73m2. This gives an average BMR of approximately 40 kcal/m2/hr. -

Approved: Tre Specific Dynamic Action of Fat and P
TRE SPECIFIC DYNAMIC ACTION OF CARBOHYD.RATF, " FAT AND P.ROJ!EIN IN FIVE WOMEN by P.:l.tricia Jo ~~IG.n.'lley Thesis submitted to the Graduate faculty of the Virginie. Fblytechnic Institute in candidacy for the degree of MAmR OF SCIENCE in RUMAN NllfRITION AND FOODS APPROVED: "Ms.cy W. Korsliind • June, 1966 Blacksburg, Virginia -2- TABLE OF CONTENTS Page ·LIST OF TABLES • • • • • • • • • • • • • • • • • • • • • • 3 LIST OF FIGURES . " . 4 ACKNOWLEDGEMENT • • . .. 5 Chapter 1. INTRODUCTION . 6 II. REVIEW OF LITERATURE • . 9 Summary of Specific Dynamic Action Theories . 9 Specific Dynamic Action of Carbohydrate . • 10 Specific Dynamic Action of Fat , • . 14 Specific Dynamic Action of Protein . .. 15 III. METHODS AND PROCEDURES . 18 Subjects . 18 Adminilt~ation of Food and Measurements . .. 19 Collection and Analysis of Expired Air • . 20 Calculations . • • . • • • . .- . .. 20 IV. RESULTS AND DISCUSSION • • • • • • . .. 21 Changes in Respiratory Quotient Following the Ingestion of Carbohydrate, Fat and Protein •• . 21 V;irr-;iations in He11t Production After Ingestion of Carbohydrate, Fat and Protein • • • 28 V. SUMMARY •••••• . 37 BIBLIOGRAPHY • . 38 VITA •• . ... 44 APPENDIX . 45 TABLE NUMBlll PAGE 1. Time required, aft•l' the inge•tion of carbohydrate by f tve women, to reach highest re•piratory quotient and highest heat production. • • • • • • • • • • • • • • • • 31 2. Maximum increaee in heat production in. four women after ingestion of fat and time required for _.ximum increase to occur •••••••••••••••••• • • 31 3. Ma;xt... tacreaee in heat production in five women after ingeatioa of protein and time required for maximwll increaee to occur. • • • • • • • • • • • •. • • • • • • • 35 -4- LIST OF FIGURES FIGURE NUMBER PAGE 1~ Changes in reepiratory quotient in five women 22 after ingestion of sucrose. -

Specific Dynamic Action
Specific Dynamic Action ➢ The phenomenon of the extra heat production by the body, over and above the calculated caloric value, when a given food is metabolized by the body, is known as specific dynamic action (SDA). ➢ It is also known as calorigenic action or thermogenic action or thermic action (effect) of food. SDA for different foods ➢ For a food containing 25 g of protein, the heat production from the caloric value is 100 Cal (25 x 4 Cal). ➢ When 25 g protein is utilized by the body, 130 Cal of heat is liberated. ➢ The extra 30 Cal is the SDA of protein. ➢ SDA for protein, fat and carbohydrate 32%, 13% & 5%, ➢ Proteins possess the highest SDA while carbohydrates have the lowest. SDA for mixed diet ➢The presence of fats and carbohydrates reduces the SDA of proteins. ➢ Fats are most efficient in reducing SDA of foodstuffs. ➢ For a regularly consumed mixed diet, the SDA is around 10%. Significance of SDA ➢ For the utilization of foods by the body, certain amount of energy is consumed from the body stores. ➢ Expenditure by the body for the utilization of foodstuffs. ➢ It is the highest for proteins (30%) and lowest for carbohydrates (5%) & for mixed diet 10%. ➢ Additional 10% calories should be added to the total energy needs (of the body) towards SDA. ➢ The higher SDA for protein indicates that it is not a good source of energy. Mechanism of SDA ➢ SDA of foods is due to the energy required for digestion, absorption, transport, metabolism and storage of foods in the body. ➢ The SDA of proteins is primarily to meet the energy requirements for deamination, synthesis of urea, biosynthesis of proteins, synthesis of triacylglycerol (from carbon skeleton of amino acids). -

Hungry Bengal: War, Famine, Riots, and the End of Empire 1939-1946
Hungry Bengal: War, Famine, Riots, and the End of Empire 1939-1946 By Janam Mukherjee A dissertation submitted in partial fulfillment of the requirement for the degree of Doctor of Philosophy (Anthropology and History) In the University of Michigan 2011 Doctoral Committee: Professor Barbara D. Metcalf, Chair Emeritus Professor David W. Cohen Associate Professor Stuart Kirsch Associate Professor Christi Merrill 1 "Unknown to me the wounds of the famine of 1943, the barbarities of war, the horror of the communal riots of 1946 were impinging on my style and engraving themselves on it, till there came a time when whatever I did, whether it was chiseling a piece of wood, or burning metal with acid to create a gaping hole, or cutting and tearing with no premeditated design, it would throw up innumerable wounds, bodying forth a single theme - the figures of the deprived, the destitute and the abandoned converging on us from all directions. The first chalk marks of famine that had passed from the fingers to engrave themselves on the heart persist indelibly." 2 Somnath Hore 1 Somnath Hore. "The Holocaust." Sculpture. Indian Writing, October 3, 2006. Web (http://indianwriting.blogsome.com/2006/10/03/somnath-hore/) accessed 04/19/2011. 2 Quoted in N. Sarkar, p. 32 © Janam S. Mukherjee 2011 To my father ii Acknowledgements I would like to thank first and foremost my father, Dr. Kalinath Mukherjee, without whom this work would not have been written. This project began, in fact, as a collaborative effort, which is how it also comes to conclusion. His always gentle, thoughtful and brilliant spirit has been guiding this work since his death in May of 2002 - and this is still our work. -

Measurement of Individual and Population Energetics of Marine Mammals
Marine Mammal Ecology and Conservation / 08-Boyd-Ch08 page 165 7:55pm OUP CORRECTED PROOF – Finals, 5/7/2010, SPi 8 Measurement of individual and population energetics of marine mammals Sara J. Iverson, Carol E. Sparling, Terrie M. Williams, Shelley L.C. Lang, and W. Don Bowen 8.1 Introduction The overriding currency of all animal life is energy. Animals have evolved strategies of energy acquisition and use, but these strategies also experience tradeoffs between energy allocated to maintenance, activities, growth, and reproduction and are central to our understanding of life histories and fitness. Thus ‘energetics’— the study of the metabolic requirements, energy use, and output of animals— underpins many areas of physiology, ecology, evolutionary, and population biology, and even ecosystem dynamics. Marine mammals pose many challenges for the study, interpretation, and com- parison of individual and population energetics. For instance, the ability to study captive animals is often restricted to a few of the smaller marine mammal species. Although opportunities in the wild may be greater, there remain serious limits to our abilities to study species in remote locations (e.g. polar bears, Ursus maritimus,in the high arctic), in unstable habitats (e.g. ice-associated pinnipeds in the Bering and Chukchi Seas), or due to endangered status (e.g. monk seals, Monachus spp., southern sea otters, Enhydra lutris nereis, and vaquita, Phocoena sinus). As a group, cetaceans pose further difficulties because of their limited accessibility and large size. Adaptive insulation (blubber) is important in temperature management (Iverson 2009a) and the presence of this comparatively inert tissue can add complexity to the issue of defining the metabolically relevant body mass in marine mammals. -

An Aspect of Nutrition and Main Food Sweeteners in the Diet
Advances in Obesity, Weight Management & Control Review Article Open Access An aspect of nutrition and main food sweeteners in the diet Abstract Volume 11 Issue 2 - 2021 Food additives are factors in both health reason and food production. Sweeteners are utilized Necla Çağlarırmak in large scale because of biochemistry, production, obesity, food structure, economy, Food Proses Department, Manisa Celal Bayar University, Turkey functional property, and research and development efforts of food industry. Intake of high calorie nutrients such as sugar in the nutrition are important factors against increasing Correspondence: Necla Çağlarırmak, Food Proses trends of obesity, cardiovascular disease and diabetes and some of chronic diseases. In Department, Manisa Celal Bayar University, Saruhanlı College, healthy nutrition, the sufficient calorie intake must be recommended for basal metabolism Saruhanlı-Manisa, Turkey, Email [email protected] and usual daily activities due to individual and environment conditions. Sweeteners are also food additives and using commonly for low calorie nutrition or from other reasons such as Received: April 01, 2021 | Published: April 12, 2021 bulk of foods. natural sweeteners can be suggested such as stevia, together with balanced and low calorie diet including vegetables, and other food types due to diet originality that change in different societies, economies, regions and education levels of people. Traditional nutrition such as Mediterranean diet can be recommended for balanced diet. This topic was reviewed under light of literature. Keywords: biochemistry, foods, JEFCA, sweeteners, obesity, nutrition, food formulas, side effects, health Abbreviations: SSBs, sugar-sweetened beverages; Ace-K, World. Nobody does not forget almost one billion people in border acesulfame potassium; ADI, adequate dietary intake of starvation or suffering the famine against obesity.2 There are lots of factors affected nutrition including economies and development Introduction strategies in the World. -

The Institutional Causes of China's Great Famine, 1959–1961
Review of Economic Studies (2015) 82, 1568–1611 doi:10.1093/restud/rdv016 © The Author 2015. Published by Oxford University Press on behalf of The Review of Economic Studies Limited. Advance access publication 20 April 2015 The Institutional Causes of China’s Great Famine, 1959–1961 Downloaded from XIN MENG Australian National University NANCY QIAN Yale University http://restud.oxfordjournals.org/ and PIERRE YARED Columbia University First version received January 2012; final version accepted January 2015 (Eds.) This article studies the causes of China’s Great Famine, during which 16.5 to 45 million individuals at Columbia University Libraries on April 25, 2016 perished in rural areas. We document that average rural food retention during the famine was too high to generate a severe famine without rural inequality in food availability; that there was significant variance in famine mortality rates across rural regions; and that rural mortality rates were positively correlated with per capita food production, a surprising pattern that is unique to the famine years. We provide evidence that an inflexible and progressive government procurement policy (where procurement could not adjust to contemporaneous production and larger shares of expected production were procured from more productive regions) was necessary for generating this pattern and that this policy was a quantitatively important contributor to overall famine mortality. Key words: Famines, Modern chinese history, Institutions, Central planning JEL Codes: P2, O43, N45 1. INTRODUCTION -

Frequently Asked Questions About Medical Foods; Second Edition Guidance for Industry
Contains Nonbinding Recommendations a v Frequently Asked Questions About Medical Foods; Second Edition Guidance for Industry Additional copies are available from: Office of Nutrition and Food Labeling, HFS-800 Center for Food Safety and Applied Nutrition Food and Drug Administration 5001 Campus Drive College Park, MD 20740 (Tel) 240-402-2373 http://www.fda.gov/FoodGuidances You may submit written comments regarding this guidance at any time. Submit electronic comments to http://www.regulations.gov. Submit written comments to the Division of Dockets Management (HFA-305), Food and Drug Administration, 5630 Fishers Lane, rm. 1061, Rockville, MD 20852. All comments should be identified with the docket number listed in the notice of availability that publishes in the Federal Register. U.S. Department of Health and Human Services Food and Drug Administration Center for Food Safety and Applied Nutrition May 2016 1 Contains Nonbinding Recommendations Table of Contents I. Introduction II. Questions and Answers III. References 2 Contains Nonbinding Recommendations Frequently Asked Questions About Medical Foods 1 Guidance for Industry This guidance represents the Food and Drug Administration’s (FDA’s) current thinking on this topic. It does not create or confer any rights for or on any person and does not operate to bind FDA or the public. You can use an alternative approach if the approach satisfies the requirements of the applicable statutes and regulations. If you want to discuss an alternative approach, contact the FDA staff responsible for implementing this guidance. If you cannot identify the appropriate FDA staff, call the telephone number listed on the title page of this guidance. -
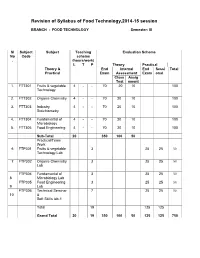
Revision of FT Syllabus Final
Revision of Syllabus of Food Technology,2014-15 session BRANCH : FOOD TECHNOLOGY Semester: III Sl Subject Subject Teaching Evaluation Scheme No Code scheme . (hours/work) L T P Theory Practical Theory & End Internal End Sessi Total Practical Exam Assessment Exam onal Class Assig Test nment 1. FTT301 Fruits & vegetable 4 - - 70 20 10 100 Technology 2. FTT302 Organic Chemistry 4 - - 70 20 10 100 3. FTT303 Industry 4 - - 70 20 10 100 Stoichiometry 4. FTT304 Fundamental of 4 - - 70 20 10 100 Microbiology 5. FTT305 Food Engineering 4 - - 70 20 10 100 Sub-Total 20 350 100 50 Practical/Team Work 6 FTP301 Fruits & vegetable 3 25 25 50 Technology Lab 7 FTP302 Organic Chemistry 3 25 25 50 Lab FTP304 Fundamental of 3 25 25 50 8 Microbiology Lab FTP305 Food Engineering 3 25 25 50 9 Lab FTP306 Technical Seminar 7 25 25 50 10 & Soft Skills lab-1 Total 19 125 125 Grand Total 20 19 350 100 50 125 125 750 FRUITS AND VEGETABLE TECHNOLOGY L T P 4 0 0 III/ FTT-301 Theory Exam. : 3 hrs. Total Contact hrs. : 60 Total Marks : 100 End Exam. : 70 Marks Theory : 60 I.A. : 20 Marks Practical : Nil Assignment : 10 Marks Objective As all the fruits & vegetable are seasonal, their storage, processing, preparation of fruits & vegetable products is highly essential. The students after completion of this paper is well concerned with the storage, preservation, processing & preparation of their products. They also will know details about the preparation of spice powder & condiment products. Topic wise distribution of periods Sl. -

Sugar Addiction
SUGAR ADDICTION BREAKING THE CYCLE DESIGNED AND PRODUCED BY: 3C Creative Agency, 3c.com.au WRITTEN BY: Constance Zhang, Platinum ILLUSTRATED BY: Andreas Samuelsson, Agent Molly & Co © 2015 Platinum Capital Limited WE ALL HAVE AN ADDICTION TO SWEET-TASTING DOPAMINE-PRODUCING SUGARY TREATS. BUT THE TREND OF INCREASING SUGAR CONSUMPTION IS RENDERED UNSUSTAINABLE BY THE ALARMING RISING RATES OF “DIABESITY”. IN THIS REPORT, WE’LL EXPLORE THE BITTERSWEET MIX OF OPPORTUNITIES. II Platinum Capital Limited Annual Report 2015 PREFACE At the first International Conference on Among the chief culprits for the so-called Nutrition held in 1992, world leaders global obesity epidemic and the sharp collectively pledged “to act in solidarity to increase in the prevalence of diabetes, ensure that freedom from hunger becomes sugar was at last recognised for what it a reality”. was, though it had been a suspect since the 1960s. At the second International Conference on Nutrition 22 years later, the It is a truth almost universally commitment changed noticeably – “to acknowledged that sugar-sweetened eradicate hunger and prevent all forms beverages are the easiest means of adding of malnutrition worldwide, particularly empty calories and gaining weight. But undernourishment, stunting, wasting, one does not need to be sipping Coca-Cola underweight and overweight in children or chewing on a favourite marzipan bar to … as well as reverse the rising trends in fall prey to sugar. From fibre-rich cereal overweight and obesity and reduce the to fat-free yogurt, from old-fashioned burden of diet-related non-communicable ketchup to exotic teriyaki sauce, one diseases in all age groups”. -

The State of Food Security and Nutrition in the World 2017
2017 THE STATE OF FOOD SECURITY AND NUTRITION IN THE WORLD BUILDING RESILIENCE FOR PEACE AND FOOD SECURITY REQUIRED CITATION: FAO, IFAD, UNICEF, WFP and WHO. 2017. The State of Food Security and Nutrition in the World 2017. Building resilience for peace and food security. Rome, FAO. The designations employed and the presentation of material in this information product do not imply the expression of any opinion whatsoever on the part of the Food and Agriculture Organization of the United Nations (FAO), the International Fund for Agricultural Development (IFAD), the United Nations Children’s Fund (UNICEF), the World Food Programme (WFP) or the World Health Organization (WHO) concerning the legal or development status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. The mention of specific companies or products of manufacturers, whether or not these have been patented, does not imply that these have been endorsed or recommended by FAO, IFAD, UNICEF, WFP or WHO in preference to others of a similar nature that are not mentioned. The designations employed and the presentation of material in the maps do not imply the expression of any opinion whatsoever on the part of FAO, IFAD, UNICEF, WFP or WHO concerning the legal or constitutional status of any country, territory or sea area, or concerning the delimitation of frontiers. All reasonable precautions have been taken by FAO, IFAD, UNICEF, WFP and WHO to verify the information contained in this publication. However, the published material is being distributed without warranty of any kind, either expressed or implied.