Artesunate + Sulfadoxine-Pyrimethamine and Artemether-Lumefantrine for Falc
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Artemether-Lumefantrine (Six-Dose Regimen) for Treating Uncomplicated Falciparum Malaria (Review)
Artemether-lumefantrine (six-dose regimen) for treating uncomplicated falciparum malaria (Review) Omari AAA, Gamble CL, Garner P This is a reprint of a Cochrane review, prepared and maintained by The Cochrane Collaboration and published in The Cochrane Library 2009, Issue 1 http://www.thecochranelibrary.com Artemether-lumefantrine (six-dose regimen) for treating uncomplicated falciparum malaria (Review) Copyright © 2009 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd. TABLE OF CONTENTS HEADER....................................... 1 ABSTRACT ...................................... 1 PLAINLANGUAGESUMMARY . 2 BACKGROUND .................................... 2 OBJECTIVES ..................................... 3 METHODS ...................................... 3 RESULTS....................................... 5 DISCUSSION ..................................... 9 AUTHORS’CONCLUSIONS . 9 ACKNOWLEDGEMENTS . 10 REFERENCES ..................................... 10 CHARACTERISTICSOFSTUDIES . 13 DATAANDANALYSES. 20 Analysis 1.1. Comparison 1 Artemether-lumefantrine vs amodiaquine, Outcome 1 Total failure by day 28. 22 Analysis 1.2. Comparison 1 Artemether-lumefantrine vs amodiaquine, Outcome 2 Total failure by day 14. 23 Analysis 1.3. Comparison 1 Artemether-lumefantrine vs amodiaquine, Outcome 3 Gametocyte carriage on day 14. 23 Analysis 2.1. Comparison 2 Artemether-lumefantrine vs chloroquine plus sulfadoxine-pyrimethamine, Outcome 1 Total failurebyday28. ................................ 24 Analysis 2.2. Comparison 2 Artemether-lumefantrine -

How to Protect Yourself Against Malaria 1 Fig
From our Whitepaper Files: How to > See companion document Protect Yourself Against Malaria World Malaria Risk Chart 2015 Edition Canada 67 Mowat Avenue, Suite 036 Toronto, Ontario M6K 3E3 (416) 652-0137 USA 1623 Military Road, #279 Niagara Falls, New York 14304-1745 (716) 754-4883 New Zealand 206 Papanui Road Christchurch 5 www.iamat.org | [email protected] | Twitter @IAMAT_Travel | Facebook IAMATHealth THE ENEMY area. Of the 460 Anopheles species, approximately 100 can transmit malaria Sunset — the hunt for human blood begins. parasites. From dusk to dawn the female Anopheles, Mosquitoes prey on a variety of hosts — the malaria-carrying mosquito searches for a host humans, monkeys, lizards, birds — carrying to supply her with blood. Blood is an absolute different species of malaria parasites which in necessity for her because it provides the protein turn infect only specific hosts. Of the approxi- needed for the development of her eggs which mately 50 different species of malaria parasites she later deposits in her breeding place. sharing the genetic name Plasmodium, only She has a tiny, elegant body, measuring 5 infect humans: Plasmodium falciparum, from 8 mm to 1 cm. She has dark spots on the killer; Plasmodium vivax; Plasmodium ovale, her wings, three pairs of long, slender legs and Plasmodium malariae and Plasmodium knowlesi. a prominent tubular proboscis with which The latter, a malaria parasite of Old World she draws blood. monkeys, has been identified to infect humans Fig. 1 Female Anopheles mosquito. The Anopheles enters your room at night. in Southeast Asia. In the past this parasite has Image source: World Health Organization You may recognize her by the way she rests been misdiagnosed as Plasmodium malariae. -

Hydroxychloroquine Or Chloroquine for Treating Coronavirus Disease 2019 (COVID-19) – a PROTOCOL for a Systematic Review of Individual Participant Data
Hydroxychloroquine or Chloroquine for treating Coronavirus Disease 2019 (COVID-19) – a PROTOCOL for a systematic review of Individual Participant Data Authors Fontes LE, Riera R, Miranda E, Oke J, Heneghan CJ, Aronson JK, Pacheco RL, Martimbianco ALC, Nunan D BACKGROUND In the face of the pandemic of SARS CoV2, urgent research is needed to test potential therapeutic agents against the disease. Reliable research shall inform clinical decision makers. Currently, there are several studies testing the efficacy and safety profiles of different pharmacological interventions. Among these drugs, we can cite antimalarial, antivirals, biological drugs, interferon, etc. As of 6 April 2020 there are three published reportsand 100 ongoing trials testing hydroxychloroquine/chloroquine alone or in association with other drugs for COVID-19. This prospective systematic review with Individual Participant data aims to assess the rigour of the best-available evidence for hydroxychloroquine or chloroquine as treatment for COVID-19 infection. The PICO framework is: P: adults with COVID-19 infection I: chloroquine or hydroxychloroquine (alone or in association) C: placebo, other active treatments, usual standard care without antimalarials O: efficacy and safety outcomes OBJECTIVES To assess the effects (benefits and harms) of chloroquine or hydroxychloroquine for the treatment of COVID-19 infection. METHODS Criteria for considering studies for this review Types of studies We shall include randomized controlled trials (RCTs) with a parallel design. We intend to include even small trials (<50 participants), facing the urgent need for evidence to respond to the current pandemic. Quasi-randomized, non-randomized, or observational studies will be excluded due to a higher risk of confounding and selection bias (1). -
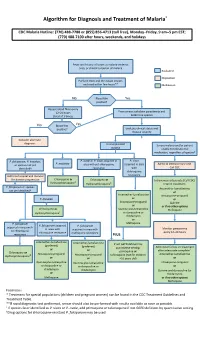
Algorithm for Diagnosis and Treatment of Malaria*
Algorithm for Diagnosis and Treatment of Malaria* CDC Malaria Hotline: (770) 488-7788 or (855) 856-4713 (toll free), Monday–Friday, 9 am–5 pm EST; (770) 488-7100 after hours, weekends, and holidays Fever and history of travel to malaria-endemic area, or clinical suspicion of malaria Evaluation Disposition Perform thick and thin blood smears and read within few hours** Medication No Blood film Yes positive? Repeat blood films every 12–24 hours From smear: calculate parasitemia and (total of 3 times) determine species No Blood film Yes positive? Evaluate clinical status and disease severity Consider alternate diagnosis Uncomplicated Severe malaria and/or patient malaria unable to tolerate oral medication, regardless of species† P. falciparum, P. knowlesi, P. ovale or P. vivax acquired in P. vivax P. malariae or species not yet area without chloroquine acquired in area Admit to intensive care unit. identified† resistance with Call CDC chloroquine Admit to hospital and monitor resistance for disease progression Chloroquine or Chloroquine or ‡ Intravenous artesunate (Call CDC) Hydroxychloroquine Hydroxychloroquine‡ Interim treatment: P. falciparum or species Artemether-lumefantrine not yet identified† or Artemether-lumefantrine Atovaquone-proguanil P. knowlesi or or Atovaquone-proguanil Quinine or or if no other options Chloroquine or Quinine plus tetracycline Mefloquine Hydroxychloroquine‡ or doxycycline or clindamycin or P. falciparum Mefloquine P. falciparum acquired P. falciparum acquired in area with in area with acquired in area with -

(Artemether / Lumefantrine 20Mg/120 Mg) Tablets
SUMMARY OF PRODUCT CHARACTERISTICS Page 1 of 13 1. NAME OF THE MEDICINAL PRODUCT Lumartem (Artemether / Lumefantrine 20mg/120 mg) Tablets 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each tablet contains: 20 mg artemether and 120 mg lumefantrine. For a full list of excipients see section 6.1. 3. PHARMACEUTICAL FORM Yellow coloured, circular, uncoated, flat faced, bevelled edged, matt finished tablets with a break line on one side and plain on the other side. The scoreline is only to facilitate breaking for ease of swallowing and not to divide into equal doses. 4. CLINICAL PARTICULARS 4.1 Therapeutic indication Artemether 20 mg and Lumefantrine 120 mg Tablets is indicated for the treatment of uncomplicated cases of malaria due to Plasmodium falciparum in adults, children and infants of 5 kg and above. The most recent official guidelines on the appropriate use of antimalarial agents and local information on the prevalence of resistance to antimalarial drugs must be taken into consideration for deciding on the appropriateness of therapy with Artemether 20 mg and Lumefantrine 120 mg Tablets. Official guidance will normally include WHO (http://whqlibdoc.who.int/publications/2010/9789241547925_eng.pdf) and local health authorities’ guidelines (see also sections 4.4 and 5.1). 4.2 Posology and method of administration Tablets for oral administration. Page 2 of 13 Number of Artemether 20 mg and Lumefantrine 120 mg Tablets for treatment according to weight bands st nd rd Weight range 1 day of 2 day of 3 day treatment treatment of treatment ≥ 5kg -

Current Antimalarial Therapies and Advances in the Development of Semi-Synthetic Artemisinin Derivatives
Anais da Academia Brasileira de Ciências (2018) 90(1 Suppl. 2): 1251-1271 (Annals of the Brazilian Academy of Sciences) Printed version ISSN 0001-3765 / Online version ISSN 1678-2690 http://dx.doi.org/10.1590/0001-3765201820170830 www.scielo.br/aabc | www.fb.com/aabcjournal Current Antimalarial Therapies and Advances in the Development of Semi-Synthetic Artemisinin Derivatives LUIZ C.S. PINHEIRO1, LÍVIA M. FEITOSA1,2, FLÁVIA F. DA SILVEIRA1,2 and NUBIA BOECHAT1 1Fundação Oswaldo Cruz, Instituto de Tecnologia em Fármacos Farmanguinhos, Fiocruz, Departamento de Síntese de Fármacos, Rua Sizenando Nabuco, 100, Manguinhos, 21041-250 Rio de Janeiro, RJ, Brazil 2Universidade Federal do Rio de Janeiro, Programa de Pós-Graduação em Química, Avenida Athos da Silveira Ramos, 149, Cidade Universitária, 21941-909 Rio de Janeiro, RJ, Brazil Manuscript received on October 17, 2017; accepted for publication on December 18, 2017 ABSTRACT According to the World Health Organization, malaria remains one of the biggest public health problems in the world. The development of resistance is a current concern, mainly because the number of safe drugs for this disease is limited. Artemisinin-based combination therapy is recommended by the World Health Organization to prevent or delay the onset of resistance. Thus, the need to obtain new drugs makes artemisinin the most widely used scaffold to obtain synthetic compounds. This review describes the drugs based on artemisinin and its derivatives, including hybrid derivatives and dimers, trimers and tetramers that contain an endoperoxide bridge. This class of compounds is of extreme importance for the discovery of new drugs to treat malaria. Key words: malaria, Plasmodium falciparum, artemisinin, hybrid. -

Malaria Treatment
TREATMENT OF MALARIA IN ADULT AND PEDIATRIC PATIENTS WHO recommendations are to treat uncomplicated malaria from all species with artemisinin-based combination therapy (such as Coartem™) based on safety and effectiveness of the drug, as well as to help streamline treatment recommendations for malaria. CDC and WHO treatment guidelines now concur with recommendations for Coartem™ as first line agent with known chloroquine resistance or species not identified. The CDC currently recommends chloroquine- containing regimens as first line therapy for chloroquine-susceptible species of malaria, which differs from WHO recommendations. We have elected to streamline institutional recommendations to be in line with WHO recommendations when there is discrepancy, given local drug availability and that Coartem™ clears parasitemia faster than chloroquine. Definitions Uncomplicated malaria: Persons with a positive blood smear OR history of recent possible exposure and no other recognized pathology who do not meet severe criteria Severe malaria: Persons with a positive blood smear OR history of recent possible exposure and no other recognized pathology who have one or more of the following clinical criteria: Parasitemia of >5% Renal failure Disseminated intravascular Jaundice Impaired Pulmonary edema coagulation Repeated generalized consciousness/coma Acute respiratory distress Spontaneous bleeding convulsions Severe normocytic syndrome Acidosis anemia Circulatory shock Hemoglobinuria Chloroquine sensitivity: P. falciparum acquired in Central America (west of the Panama Canal), Haiti, and Dominican Republic All P. malariae, P. knowlesi, and P. ovale All P. vivax EXCEPT infections acquired in Papua New Guinea or Indonesia Uncomplicated malaria P. falciparum or not identified P. malariae or P. knowlesi P. vivax or P. ovale chloroquine sensitive P. -

Hydroxychloroquine (All Populations Monograph)
3/17/2020 Clinical Pharmacology powered by ClinicalKey Hydroxychloroquine (All Populations Monograph) Indications/Dosage expand all | collapse all Labeled malaria rheumatoid arthritis malaria prophylaxis systemic lupus erythematosus (SLE) Off-Label, Recommended coronavirus disease 2019 (COVID-19) † severe acute respiratory syndrome coronavirus lupus nephritis † 2 (SARS-CoV-2) infection † polymorphous light eruption † † Off-label indication Per the manufacturer, this drug has been shown to be active against most strains of the following microorganisms either in vitro and/or in clinical infections: Plasmodium falciparum, Plasmodium malariae, Plasmodium ovale, Plasmodium vivax. NOTE: The safety and effectiveness in treating clinical infections due to organisms with in vitro data only have not been established in adequate and well-controlled clinical trials. This drug may also have activity against the following microorganisms: severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). NOTE: Some organisms may not have been adequately studied during clinical trials; therefore, exclusion from this list does not necessarily negate the drug’s activity against the organism. For the treatment of malaria due to susceptible strains of P. falciparum, P. knowlesi†, P. malariae, P. ovale, and P. vivax https://www.clinicalkey.com/pharmacology/monograph/print?cpnum=300&type=0&printSections=monindi&printSections=monsup&printSections=mon… 1/87 3/17/2020 Clinical Pharmacology powered by ClinicalKey Oral dosage Adults 13 mg (10 mg base)/kg/dose [Max: 800 mg (620 mg base)/dose] PO, then 6.5 mg (5 mg base)/kg/dose [Max: 400 mg (310 mg base)/dose] PO at 6, 24, and 48 hours after the initial dose.[41806] [64059] For P. -

Guidelines for Treatment of Malaria in the United States 1 (Based on Drugs Currently Available for Use in the United States — October 1, 2019)
Guidelines for Treatment of Malaria in the United States 1 (Based on drugs currently available for use in the United States — October 1, 2019) CDC Malaria Hotline: (770) 488-7788 or (855) 856-4713 (toll free) Monday–Friday, 9 am to 5 pm EST; (770) 488-7100 after hours, weekends, and holidays Clinical Diagnosis/ Drug Susceptibility (Based on Recommended Regimen and Adult Dose1 Recommended Regimen and Pediatric Dose1 Plasmodium Species Region Infection Was Acquired) Pediatric dose should NEVER exceed adult dose Uncomplicated malaria/ Chloroquine resistance or unknown A. Artemether-lumefantrine (Coartem™)3,4 P. falciparum, or resistance2 Tablet=20mg artemether/ 120 mg lumefantrine species not identified All malarious regions except those specified A 3-day treatment schedule with a total of 6 oral doses is recommended for both adult and pediatric patients based on as chloroquine sensitive listed in the box weight. The patient should receive the initial dose, followed by the second dose 8 hours later, then 1 dose bid for the If “species not identified” below following 2 days. Dosing as follows: is later diagnosed as P. 5–<15 kg: 1 tablet per dose vivax or P. ovale, please 15–<25 kg: 2 tablets per dose see P. vivax and P. ovale 25–<35 kg: 3 tablets per dose (below) re: treatment with ≥35 kg: 4 tablets per dose primaquine or tafenoquine B. Atovaquone-proguanil (Malarone™)4,5 B. Atovaquone-proguanil (Malarone™)4,5 Adult tablet= 250 mg atovaquone/ 100 mg proguanil Adult tab=250 mg atovaquone/ 100 mg proguanil 4 adult tabs po qd x 3 days Peds tab=62.5 mg atovaquone/ 25 mg proguanil 5–<8 kg: 2 peds tabs po qd x 3 days 8–<10 kg: 3 peds tabs po qd x 3 days 10–<20 kg: 1 adult tab po qd x 3 days 20–<30 kg: 2 adult tabs po qd x 3 days 30–<40 kg: 3 adult tabs po qd x 3 days ≥40 kg: 4 adult tabs po qd x 3 days C. -

Common Study Protocol for Observational Database Studies WP5 – Analytic Database Studies
Arrhythmogenic potential of drugs FP7-HEALTH-241679 http://www.aritmo-project.org/ Common Study Protocol for Observational Database Studies WP5 – Analytic Database Studies V 1.3 Draft Lead beneficiary: EMC Date: 03/01/2010 Nature: Report Dissemination level: D5.2 Report on Common Study Protocol for Observational Database Studies WP5: Conduct of Additional Observational Security: Studies. Author(s): Gianluca Trifiro’ (EMC), Giampiero Version: v1.1– 2/85 Mazzaglia (F-SIMG) Draft TABLE OF CONTENTS DOCUMENT INFOOMATION AND HISTORY ...........................................................................4 DEFINITIONS .................................................... ERRORE. IL SEGNALIBRO NON È DEFINITO. ABBREVIATIONS ......................................................................................................................6 1. BACKGROUND .................................................................................................................7 2. STUDY OBJECTIVES................................ ERRORE. IL SEGNALIBRO NON È DEFINITO. 3. METHODS ..........................................................................................................................8 3.1.STUDY DESIGN ....................................................................................................................8 3.2.DATA SOURCES ..................................................................................................................9 3.2.1. IPCI Database .....................................................................................................9 -

Antiparasitic Phytotherapy Perspectives, Scope and Current Development
REVISIÓN DE TEMA Antiparasitic phytotherapy perspectives, scope and current development Jhon Carlos Castaño Osorio1,*, Alejandra María Giraldo García1 Abstract Tropical protozoan diseases are currently a major public health problem throughout the world and are strongly linked with poverty, this combined with a lack of commercial markets for potential drugs has created a large burden on the health and economic development of low-income and middle-income countries in Africa, Asia, and the Americas. Due to the low research interest and the high increase of resistance against the existing treatments, as well as increasing inefficiency, toxicity, prolonged treatment schedules and costs, there is an urgent need for cost-effective, safe and easy-to-administer, new effective compounds with novel mechanisms of action. Several studies of crude plant extracts have already identified potential compounds to treat Chagas’ disease, Leishmaniasis, Toxoplasmosis, Giardiasis, and Malaria among other protozoan parasites. Natural compounds of medicinal plants have shown lower toxicity together with higher specificity, crea- ting an optimistic view of new treatments for diseases. Out of 1010 new active substances approved as drugs for medical conditions by regulatory agencies during the past 25 years, 490(48.5%) were from a natural origin. Keywords: Antiparasitic Agents, Phytotherapy, Biological Products, Malaria, Trypanosoma, Leishmania, Toxoplasma, Amoeba. Perspectivas, enfoque y actual desarrollo de la fitoterapia antiparasitaria Resumen Las enfermedades tropicales por protozoarios son un problema importante de salud pública en todo el mundo y están ligadas fuertemente con la pobreza. Esto lleva a que falte investigación por las empresas farmacéuticas con ánimo de lucro, que sólo se motivan por posibilidades de mercado. -

Anti-Malaria Drug Policy for Ghana
ANTI-MALARIA DRUG POLICY FOR GHANA REPUBLIC OF GHANA ANTI-MALARIA DRUG POLICY FOR GHANA ANTI-MALARIA DRUG POLICY FOR GHANA Ministry of Health 1st Revised Version 2007 2nd Revised Version 2009 MINISTRY OF HEALTH ANTI-MALARIA DRUG POLICY FOR GHANA TABLE OF CONTENTS Preface iv Acknowledgement v Working Group vii Introduction 1 Situation Analysis 3 Policy Change for Drug Use in Malaria 7 Chemoprophylaxis of Malaria in Pregnancy 12 Malaria Prophylaxis in Non Immunes 13 Steps Involved in the Change Process 15 Capacity Building and Public Education 19 Monitoring and Evaluation 21 Standards and Regulation 22 MINISTRY OF HEALTH I ANTI-MALARIA DRUG POLICY FOR GHANA LIST OF ABBREVIATIONS ACT Artemisinin based combination therapy AMDP Anti-Malaria Drug Policy ANCs Antenatal Clinics BCC Behaviour Change Communication CBA Community-Based Agent CBHWs Community-Based Health Workers CHIM Centre for Health Information Management DFID Department for International Development DHMT District Health Management Team DOT Directly Observed Therapy EPI Expanded Programme on Immunisation FDB Food and Drugs Board G6PD Glucose-6 phosphate dehydrogenase GHS Ghana Health Service GoG Government of Ghana HBC Home Based Care HIS Health Information System HPU Health Promotion Unit HRU Health Research Unit IEC Information, Education and Communication IMCI Integrated Management of Childhood Illnesses IPT Intermittent Presumptive Treatment ITMs Insecticide Treated Materials ITNs Insecticide Treated Nets JICA Japan International Coorporation Agency KABP Knowledge, Attitude,