(Artemether / Lumefantrine 20Mg/120 Mg) Tablets
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Trypanocidal Activity of Ethanolic Leaf Extract of Andrographis Paniculata
Science World Journal Vol. 15(No 4) 2020 www.scienceworldjournal.org ISSN: 1597-6343 (Online), ISSN: 2756-391X (Print) Published by Faculty of Science, Kaduna State University TRYPANOCIDAL ACTIVITY OF ETHANOLIC LEAF EXTRACT OF ANDROGRAPHIS PANICULATA 1* 2 3 4 Ladan Z., Olanrewaju T. O., Maikaje D.B., and Waziri P. N. Full Length Research Article 1Department of Chemistry, Kaduna State University, Kaduna, Nigeria 2Human African Trypanosomiasis Research Department, Nigerian Institute for Trypanosomiasis Research, Kaduna, Nigeria 3Department of Microbiology, Kaduna State University, Kaduna, Nigeria 4Department of Biochemistry, Kaduna State University, Kaduna, Nigeria *Corresponding Author’s Email Address: [email protected] Phone: 2347030475898 ABSTRACT animal Trypanosomiasis (Achenef and Bekele, 2013; Giordani et Andrographis paniculata used in this study belongs to Acanthacae al., 2016). Various industries are now on the search for sources of family and is commonly known as king of bitters. This study aimed alternative which include synthetic and natural products. Medicinal at evaluating effect of A. paniculata leaf extract on experimental plants remain a major source of alternative medicine and scientific rats infected with Trypanosoma brucei brucei. Cold maceration was investigations have shown that various plants indicate promising used for extraction and the biomass was fractionated in open results for the development of cheaper less toxic drugs for column chromatography. One of the ethanolic fractions obtained treatment and management of trypanosomiasis (Simoben et al., from A. paniculata was evaluated for in vitro and in vivo activities 2018). Andrographis paniculata evaluated in this study belong to using RPMI 1640 cell culture media and experimental wistar rats Acanthacae family of the plant kingdom and commonly known as respectively. -

Pharmaceutical Composition Comprising Artesunate
(19) TZZ ¥ 4B_T (11) EP 2 322 244 B1 (12) EUROPEAN PATENT SPECIFICATION (45) Date of publication and mention (51) Int Cl.: of the grant of the patent: A61P 33/06 (2006.01) A61K 9/20 (2006.01) 21.11.2012 Bulletin 2012/47 A61K 31/357 (2006.01) A61K 31/505 (2006.01) A61K 31/4965 (2006.01) (21) Application number: 10192206.0 (22) Date of filing: 09.09.2005 (54) Pharmaceutical composition comprising artesunate, sulfamethoxypyrazine and pyrimethamine suitable for the treatment of malaria within one day, in the form of a tablet Pharmazeutische Zusammensetzung enthaltend Artesunat, Sulfamethoxypyrazin und Pyrimethamin, die zur Behandlung von Malaria innerhalb eines Tages geeignet ist, in Form einer Tablette Composition pharmaceutique à base d’artesunate, de sulfaméthoxypyrazine et de pyriméthamine permettant le traitement du palludisme en un jour, sous forme de comprimé (84) Designated Contracting States: (56) References cited: AT BE BG CH CY CZ DE DK EE ES FI FR GB GR • LUXEMBURGER C ET AL: "Single day HU IE IS IT LI LT LU LV MC NL PL PT RO SE SI mefloquine-artesunate combination in the SK TR treatment of multi-drug resistant falciparum malaria", TRANSACTIONS OF THE ROYAL (43) Date of publication of application: SOCIETY OF TROPICAL MEDICINE AND 18.05.2011 Bulletin 2011/20 HYGIENE, ELSEVIER, GB, vol. 88, no. 2, 1 March 1994 (1994-03-01), pages 213-217, XP023097868, (62) Document number(s) of the earlier application(s) in ELSEVIER, GB ISSN: 0035-9203, DOI: accordance with Art. 76 EPC: 10.1016/0035-9203(94)90303-4 [retrieved on 05795966.0 / 1 940 519 1994-03-01] • DAO ET AL: "Fatty food does not alter blood (73) Proprietor: Dafra Pharma N.V. -

Artemether-Lumefantrine (Six-Dose Regimen) for Treating Uncomplicated Falciparum Malaria (Review)
Artemether-lumefantrine (six-dose regimen) for treating uncomplicated falciparum malaria (Review) Omari AAA, Gamble CL, Garner P This is a reprint of a Cochrane review, prepared and maintained by The Cochrane Collaboration and published in The Cochrane Library 2009, Issue 1 http://www.thecochranelibrary.com Artemether-lumefantrine (six-dose regimen) for treating uncomplicated falciparum malaria (Review) Copyright © 2009 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd. TABLE OF CONTENTS HEADER....................................... 1 ABSTRACT ...................................... 1 PLAINLANGUAGESUMMARY . 2 BACKGROUND .................................... 2 OBJECTIVES ..................................... 3 METHODS ...................................... 3 RESULTS....................................... 5 DISCUSSION ..................................... 9 AUTHORS’CONCLUSIONS . 9 ACKNOWLEDGEMENTS . 10 REFERENCES ..................................... 10 CHARACTERISTICSOFSTUDIES . 13 DATAANDANALYSES. 20 Analysis 1.1. Comparison 1 Artemether-lumefantrine vs amodiaquine, Outcome 1 Total failure by day 28. 22 Analysis 1.2. Comparison 1 Artemether-lumefantrine vs amodiaquine, Outcome 2 Total failure by day 14. 23 Analysis 1.3. Comparison 1 Artemether-lumefantrine vs amodiaquine, Outcome 3 Gametocyte carriage on day 14. 23 Analysis 2.1. Comparison 2 Artemether-lumefantrine vs chloroquine plus sulfadoxine-pyrimethamine, Outcome 1 Total failurebyday28. ................................ 24 Analysis 2.2. Comparison 2 Artemether-lumefantrine -

Halfanâ Brand of Halofantrine Hydrochloride Tablets
HL:L4 PRESCRIBING INFORMATION HALFANâ brand of halofantrine hydrochloride Tablets WARNING: HALFAN HAS BEEN SHOWN TO PROLONG QTc INTERVAL AT THE RECOMMENDED THERAPEUTIC DOSE. THERE HAVE BEEN RARE REPORTS OF SERIOUS VENTRICULAR DYSRHYTHMIAS SOMETIMES ASSOCIATED WITH DEATH, WHICH MAY BE SUDDEN. HALFAN IS THEREFORE NOT RECOMMENDED FOR USE IN COMBINATION WITH DRUGS OR CLINICAL CONDITIONS KNOWN TO PROLONG QTc INTERVAL, OR IN PATIENTS WHO HAVE PREVIOUSLY RECEIVED MEFLOQUINE, OR IN PATIENTS WITH KNOWN OR SUSPECTED VENTRICULAR DYSRHYTHMIAS, A-V CONDUCTION DISORDERS OR UNEXPLAINED SYNCOPAL ATTACKS. HALFAN SHOULD BE PRESCRIBED ONLY BY PHYSICIANS WHO HAVE SPECIAL COMPETENCE IN THE DIAGNOSIS AND TREATMENT OF MALARIA, AND WHO ARE EXPERIENCED IN THE USE OF ANTIMALARIAL DRUGS. PHYSICIANS SHOULD THOROUGHLY FAMILIARIZE THEMSELVES WITH THE COMPLETE CONTENTS OF THIS LEAFLET BEFORE PRESCRIBING HALFAN. DESCRIPTION Halfan (halofantrine hydrochloride) is an antimalarial drug available as tablets containing 250 mg of halofantrine hydrochloride (equivalent to 233 mg of the free base) for oral administration. The chemical name of halofantrine hydrochloride is 1,3-dichloro-a-[2-(dibutylamino) ethyl]- 6-(trifluoromethyl)-9-phenanthrene-methanol hydrochloride. The drug, a white to off-white crystalline compound, is practically insoluble in water. Halofantrine hydrochloride has a calculated molecular weight of 536.89. The empirical formula is C26H30Cl2F3NOHCl and the structural formula is 1 Inactive Ingredients Inactive ingredients are magnesium stearate, microcrystalline cellulose, povidone, pregelatinized starch, sodium starch glycolate and talc. CLINICAL PHARMACOLOGY The interindividual variability in the pharmacokinetic parameters of halofantrine is very wide and has led to great difficulty in precisely determining the pharmacokinetic characteristics of this product. Following administration of halofantrine hydrochloride tablets in single oral doses of 250 mg to 1000 mg to healthy volunteers, peak plasma levels were reached in 5 to 7 hours. -

How to Protect Yourself Against Malaria 1 Fig
From our Whitepaper Files: How to > See companion document Protect Yourself Against Malaria World Malaria Risk Chart 2015 Edition Canada 67 Mowat Avenue, Suite 036 Toronto, Ontario M6K 3E3 (416) 652-0137 USA 1623 Military Road, #279 Niagara Falls, New York 14304-1745 (716) 754-4883 New Zealand 206 Papanui Road Christchurch 5 www.iamat.org | [email protected] | Twitter @IAMAT_Travel | Facebook IAMATHealth THE ENEMY area. Of the 460 Anopheles species, approximately 100 can transmit malaria Sunset — the hunt for human blood begins. parasites. From dusk to dawn the female Anopheles, Mosquitoes prey on a variety of hosts — the malaria-carrying mosquito searches for a host humans, monkeys, lizards, birds — carrying to supply her with blood. Blood is an absolute different species of malaria parasites which in necessity for her because it provides the protein turn infect only specific hosts. Of the approxi- needed for the development of her eggs which mately 50 different species of malaria parasites she later deposits in her breeding place. sharing the genetic name Plasmodium, only She has a tiny, elegant body, measuring 5 infect humans: Plasmodium falciparum, from 8 mm to 1 cm. She has dark spots on the killer; Plasmodium vivax; Plasmodium ovale, her wings, three pairs of long, slender legs and Plasmodium malariae and Plasmodium knowlesi. a prominent tubular proboscis with which The latter, a malaria parasite of Old World she draws blood. monkeys, has been identified to infect humans Fig. 1 Female Anopheles mosquito. The Anopheles enters your room at night. in Southeast Asia. In the past this parasite has Image source: World Health Organization You may recognize her by the way she rests been misdiagnosed as Plasmodium malariae. -

Hydroxychloroquine Or Chloroquine for Treating Coronavirus Disease 2019 (COVID-19) – a PROTOCOL for a Systematic Review of Individual Participant Data
Hydroxychloroquine or Chloroquine for treating Coronavirus Disease 2019 (COVID-19) – a PROTOCOL for a systematic review of Individual Participant Data Authors Fontes LE, Riera R, Miranda E, Oke J, Heneghan CJ, Aronson JK, Pacheco RL, Martimbianco ALC, Nunan D BACKGROUND In the face of the pandemic of SARS CoV2, urgent research is needed to test potential therapeutic agents against the disease. Reliable research shall inform clinical decision makers. Currently, there are several studies testing the efficacy and safety profiles of different pharmacological interventions. Among these drugs, we can cite antimalarial, antivirals, biological drugs, interferon, etc. As of 6 April 2020 there are three published reportsand 100 ongoing trials testing hydroxychloroquine/chloroquine alone or in association with other drugs for COVID-19. This prospective systematic review with Individual Participant data aims to assess the rigour of the best-available evidence for hydroxychloroquine or chloroquine as treatment for COVID-19 infection. The PICO framework is: P: adults with COVID-19 infection I: chloroquine or hydroxychloroquine (alone or in association) C: placebo, other active treatments, usual standard care without antimalarials O: efficacy and safety outcomes OBJECTIVES To assess the effects (benefits and harms) of chloroquine or hydroxychloroquine for the treatment of COVID-19 infection. METHODS Criteria for considering studies for this review Types of studies We shall include randomized controlled trials (RCTs) with a parallel design. We intend to include even small trials (<50 participants), facing the urgent need for evidence to respond to the current pandemic. Quasi-randomized, non-randomized, or observational studies will be excluded due to a higher risk of confounding and selection bias (1). -

Quinoline-Based Hybrid Compounds with Antimalarial Activity
molecules Review Quinoline-Based Hybrid Compounds with Antimalarial Activity Xhamla Nqoro, Naki Tobeka and Blessing A. Aderibigbe * Department of Chemistry, University of Fort Hare, Alice Campus, Alice 5700, Eastern Cape, South Africa; [email protected] (X.N.); [email protected] (N.T.) * Correspondence: [email protected] Received: 7 November 2017; Accepted: 11 December 2017; Published: 19 December 2017 Abstract: The application of quinoline-based compounds for the treatment of malaria infections is hampered by drug resistance. Drug resistance has led to the combination of quinolines with other classes of antimalarials resulting in enhanced therapeutic outcomes. However, the combination of antimalarials is limited by drug-drug interactions. In order to overcome the aforementioned factors, several researchers have reported hybrid compounds prepared by reacting quinoline-based compounds with other compounds via selected functionalities. This review will focus on the currently reported quinoline-based hybrid compounds and their preclinical studies. Keywords: 4-aminoquinoline; 8-aminoquinoline; malaria; infectious disease; hybrid compound 1. Introduction Malaria is a parasitic infectious disease that is a threat to approximately half of the world’s population. This parasitic disease is transmitted to humans by a female Anopheles mosquito when it takes a blood meal. Pregnant women and young children in the sub-tropical regions are more prone to malaria infection. In 2015, 214 million infections were reported, with approximately 438,000 deaths and the African region accounted for 90% of the deaths [1–3]. Malaria is caused by five parasites of the genus Plasmodium, but the majority of deaths are caused by Plasmodium falciparum and Plasmodium vivax [1,4–6]. -

Part 4 May 2016 Sulfadoxine/Pyrimethamine 500Mg/25Mg, Tablets, (Guilin Pharmaceutical Co., Ltd), MA066
Artesunate 50mg Tablets + WHOPAR part 4 May 2016 Sulfadoxine/Pyrimethamine 500mg/25mg, Tablets, (Guilin Pharmaceutical Co., Ltd), MA066 SUMMARY OF PRODUCT CHARACTERISTICS 1 Artesunate 50mg Tablets + WHOPAR part 4 May 2016 Sulfadoxine/Pyrimethamine 500mg/25mg, Tablets, (Guilin Pharmaceutical Co., Ltd), MA066 1. NAME OF THE MEDICINAL PRODUCT ARTECOSPE ® 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each artesunate tablet contains 50 mg artesunate, each sulfadoxine/pyrimethamine tablet contains 500 mg sulfadoxine and 25 mg pyrimethamine. Each artesunate tablet contains 15 mg of sucrose. Each sulfadoxine/pyrimethamine tablet contains 20 mg of lactose. For a full list of excipients, see section 6.1. 3. PHARMACEUTICAL FORM The artesunate 50 mg tablets are white round tablets debossed with “AS ” on one side and a score line on the other side. The sulfadoxine/pyrimethamine tablets (500/25 mg) are white, round, and debossed with “SP” on both sides, with a score line on one side. 4. CLINICAL PARTICULARS 4.1 Therapeutic indication ARTECOSPE® (artesunate + sulfadoxine/pyrimethamine) is indicated for the treatment of uncomplicated cases of malaria due to Plasmodium falciparum strains which are susceptible to artesunate as well as to sulfadoxine /pyrimethamine. The most recent official guidelines on the appropriate use of antimalarial agents and local information on the prevalence of resistance to antimalarial drugs must be taken into consideration for deciding on the appropriateness of therapy with artesunate tablets and sulfadoxine/pyrimethamine tablets. Official guidance will normally include WHO (http://apps.who.int/iris/bitstream/10665/162441/1/9789241549127_eng.pdf?ua=1&ua=1) and public health authorities’ guidelines (see also sections 4.4 and 5.1). -

The History and Ethics of Malaria Eradication and Control Campaigns in Tropical Africa
Malaria Redux: The History and Ethics of Malaria Eradication and Control Campaigns in Tropical Africa Center for Historical Research Ohio State University Spring 2012 Seminars: Epidemiology in World History Prof. J.L.A. Webb, Jr. Department of History Colby College DRAFT: NOT FOR CITATION 2 During the 1950s, colonial malariologists, in conjunction with experts from the World Health Organization (WHO), set up malaria eradication pilot projects across tropical Africa. They deployed new synthetic insecticides such as DLD, HCH, and DDT, and new antimalarials, such as chloroquine and pyrimethamine, in an effort to establish protocols for eradication. These efforts ‘protected’ some fourteen million Africans. Yet by the early 1960s, the experts concluded that malaria eradication was not feasible, and the pilot projects were disbanded. The projects had achieved extremely low levels of infection for years at a time, but the experts had to accept with regret that their interventions were unable to reduce malaria transmission to zero. The projects had high recurrent costs, and it was understood that they were financially unsustainable. The pilot projects were allowed to lapse. The malaria eradication pilot projects had reduced the rates of infection to levels so low that the ‘protected’ populations lost their acquired immunities to malaria during the years of the projects. In the immediate aftermath of the projects, the Africans were subject to severe malaria, which sometimes afflicted entire communities in epidemic form, until they regained their immunities. How Should We Understand the Ethics of the Early Eradication Efforts? The ethics of malaria control in the 1950s and 1960s seemed self-evident to the interventionists. -

Prevention of Malaria in Travellers (Pdf 59KB)
THEME Travel medicine Prevention of malaria in travellers BACKGROUND Malaria remains endemic in over 100 countries worldwide. Travellers to these countries may be at risk of contracting disease. Assessing risk on an individual basis can be challenging. Trish Batchelor OBJECTIVE MBBS, MPH, FRACGP, is This article identifies the traveller at high risk of contracting malaria, outlines preventive methods, including personal Medical Director, Travel Doctor- protection and chemoprophylaxis if indicated, and summarises the risks and benefits of the most commonly used TMVC, Canberra, Australian chemoprophylactic agents. Capital Territory, and Executive Board Member, International DISCUSSION Society of Travel Medicine. Appropriately assessing the risk of malaria in an individual traveller can be complex. A number of factors beyond the trish.batchelor@traveldoctor. com.au country being visited influence the level of risk. These should be identified and taken into account when discussing malaria prevention with individuals. The traveller should be actively involved in the decision making process in order Tony Gherardin to enhance compliance. High risk individuals should be identified. Personal protection methods should always be MBBS, MPH, FRACGP, is emphasised. If chemoprophylaxis is indicated, the contraindications, advantages and side effects should be discussed. National Medical Adviser, Travel Doctor-TMVC, If in doubt, referral to a specialised travel medicine clinic should be considered. Melbourne, Victoria. The malaria parasite, spread via the bite of an infected Risk for travellers female Anopheles mosquito, remains a significant health threat in over 100 countries worldwide. An The risk of contracting malaria varies significantly from estimated 350–500 million cases occur annually, with traveller to traveller, and this is the challenge of providing over a million sub-Saharan Africans, mainly children, quality advice. -

Estonian Statistics on Medicines 2016 1/41
Estonian Statistics on Medicines 2016 ATC code ATC group / Active substance (rout of admin.) Quantity sold Unit DDD Unit DDD/1000/ day A ALIMENTARY TRACT AND METABOLISM 167,8985 A01 STOMATOLOGICAL PREPARATIONS 0,0738 A01A STOMATOLOGICAL PREPARATIONS 0,0738 A01AB Antiinfectives and antiseptics for local oral treatment 0,0738 A01AB09 Miconazole (O) 7088 g 0,2 g 0,0738 A01AB12 Hexetidine (O) 1951200 ml A01AB81 Neomycin+ Benzocaine (dental) 30200 pieces A01AB82 Demeclocycline+ Triamcinolone (dental) 680 g A01AC Corticosteroids for local oral treatment A01AC81 Dexamethasone+ Thymol (dental) 3094 ml A01AD Other agents for local oral treatment A01AD80 Lidocaine+ Cetylpyridinium chloride (gingival) 227150 g A01AD81 Lidocaine+ Cetrimide (O) 30900 g A01AD82 Choline salicylate (O) 864720 pieces A01AD83 Lidocaine+ Chamomille extract (O) 370080 g A01AD90 Lidocaine+ Paraformaldehyde (dental) 405 g A02 DRUGS FOR ACID RELATED DISORDERS 47,1312 A02A ANTACIDS 1,0133 Combinations and complexes of aluminium, calcium and A02AD 1,0133 magnesium compounds A02AD81 Aluminium hydroxide+ Magnesium hydroxide (O) 811120 pieces 10 pieces 0,1689 A02AD81 Aluminium hydroxide+ Magnesium hydroxide (O) 3101974 ml 50 ml 0,1292 A02AD83 Calcium carbonate+ Magnesium carbonate (O) 3434232 pieces 10 pieces 0,7152 DRUGS FOR PEPTIC ULCER AND GASTRO- A02B 46,1179 OESOPHAGEAL REFLUX DISEASE (GORD) A02BA H2-receptor antagonists 2,3855 A02BA02 Ranitidine (O) 340327,5 g 0,3 g 2,3624 A02BA02 Ranitidine (P) 3318,25 g 0,3 g 0,0230 A02BC Proton pump inhibitors 43,7324 A02BC01 Omeprazole -
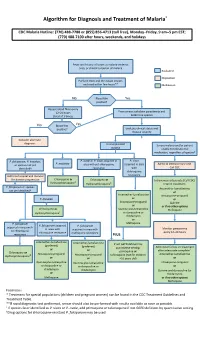
Algorithm for Diagnosis and Treatment of Malaria*
Algorithm for Diagnosis and Treatment of Malaria* CDC Malaria Hotline: (770) 488-7788 or (855) 856-4713 (toll free), Monday–Friday, 9 am–5 pm EST; (770) 488-7100 after hours, weekends, and holidays Fever and history of travel to malaria-endemic area, or clinical suspicion of malaria Evaluation Disposition Perform thick and thin blood smears and read within few hours** Medication No Blood film Yes positive? Repeat blood films every 12–24 hours From smear: calculate parasitemia and (total of 3 times) determine species No Blood film Yes positive? Evaluate clinical status and disease severity Consider alternate diagnosis Uncomplicated Severe malaria and/or patient malaria unable to tolerate oral medication, regardless of species† P. falciparum, P. knowlesi, P. ovale or P. vivax acquired in P. vivax P. malariae or species not yet area without chloroquine acquired in area Admit to intensive care unit. identified† resistance with Call CDC chloroquine Admit to hospital and monitor resistance for disease progression Chloroquine or Chloroquine or ‡ Intravenous artesunate (Call CDC) Hydroxychloroquine Hydroxychloroquine‡ Interim treatment: P. falciparum or species Artemether-lumefantrine not yet identified† or Artemether-lumefantrine Atovaquone-proguanil P. knowlesi or or Atovaquone-proguanil Quinine or or if no other options Chloroquine or Quinine plus tetracycline Mefloquine Hydroxychloroquine‡ or doxycycline or clindamycin or P. falciparum Mefloquine P. falciparum acquired P. falciparum acquired in area with in area with acquired in area with