Antiparasitic Phytotherapy Perspectives, Scope and Current Development
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Aspects of Physiology and Trichome Chemistry in the Medicinal Plant
Aspects of Physiology and Trichome Chemistry in the Medicinal Plant Tanacetum parthenium (L.) Schultz-Bip. by Kevin Bernard Usher B.Sc, Okanagan University College, 1994 A THESIS SUBMITTED IN PARTIAL FULLFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF DOCTOR OF PHILOSOPHY in THE FACULTY OF GRADUATE STUDIES DEPARTMENT OF BOTANY We accept this thesis as conforming to the required standard G.H.N. Towers, Supervisor (Botany, University of British Columbia) .E.P. Taylor, Co^ipen/isor (Botany, University of British Columbia) P.A. Bowen, Committee Member (Pacific Agriculture Research Center, Agriculture and Agri-Food Canada) A.D./vKala^s, Commit$e4v1ember (Botany, University of British Columbia) THE UNIVERSITY OF BRITISH COLUMBIA September 2001 © Kevin Bernard Usher, 2001 In presenting this thesis in partial fulfilment of the requirements for an advanced degree at the University of British Columbia, I agree that the Library shall make it freely available for reference and study. I further agree that permission for extensive copying of this thesis for scholarly purposes may be granted by the head of my department or by his or her representatives. It is understood that copying or publication of this thesis for financial gain shall not be allowed without my written permission. Department The University of British Columbia Vancouver, Canada Date S" Oct , Zoo( DE-6 (2/88) 11 ABSTRACT This study investigated aspects of physiology and terpenoid chemistry in feverfew, a medicinal plant used for migraine therapy. The sesquiterpene lactone parthenolide accumulates in feverfew shoots and is thought to contribute to feverfew's antimigraine activity. The first part of this study examined the effects of nitrogen application and irrigation on shoot yield and shoot parthenolide concentration. -

Folic Acid Antagonists: Antimicrobial and Immunomodulating Mechanisms and Applications
International Journal of Molecular Sciences Review Folic Acid Antagonists: Antimicrobial and Immunomodulating Mechanisms and Applications Daniel Fernández-Villa 1, Maria Rosa Aguilar 1,2 and Luis Rojo 1,2,* 1 Instituto de Ciencia y Tecnología de Polímeros, Consejo Superior de Investigaciones Científicas, CSIC, 28006 Madrid, Spain; [email protected] (D.F.-V.); [email protected] (M.R.A.) 2 Consorcio Centro de Investigación Biomédica en Red de Bioingeniería, Biomateriales y Nanomedicina, 28029 Madrid, Spain * Correspondence: [email protected]; Tel.: +34-915-622-900 Received: 18 September 2019; Accepted: 7 October 2019; Published: 9 October 2019 Abstract: Bacterial, protozoan and other microbial infections share an accelerated metabolic rate. In order to ensure a proper functioning of cell replication and proteins and nucleic acids synthesis processes, folate metabolism rate is also increased in these cases. For this reason, folic acid antagonists have been used since their discovery to treat different kinds of microbial infections, taking advantage of this metabolic difference when compared with human cells. However, resistances to these compounds have emerged since then and only combined therapies are currently used in clinic. In addition, some of these compounds have been found to have an immunomodulatory behavior that allows clinicians using them as anti-inflammatory or immunosuppressive drugs. Therefore, the aim of this review is to provide an updated state-of-the-art on the use of antifolates as antibacterial and immunomodulating agents in the clinical setting, as well as to present their action mechanisms and currently investigated biomedical applications. Keywords: folic acid antagonists; antifolates; antibiotics; antibacterials; immunomodulation; sulfonamides; antimalarial 1. -
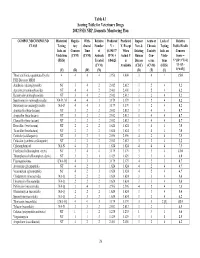
2002 FSIS National Residue Program, Section 4
Table 4.1 Scoring Table for Veterinary Drugs 2002 FSIS NRP, Domestic Monitoring Plan COMPOUND/COMPOUND Historical Regula- With- Relative Predicted Predicted Impact Acute or Lack of Relative CLASS Testing tory drawal Number V = V, Except New & Chronic Testing Public Health Info. on Concern Time of (0.19437* When Existing Toxicity Info. on Concern Violations (CVM) (CVM) Animals R*N) + Actual V Human Con- Viola- Score = (FSIS) Treated 0.84625 is Disease cerns tions V*[(D+3*T)/4] (CVM) Available (CDC) (CVM) (FSIS) *{1+[(L- (V) (R) (W) (N) (D) (T) (L) 1)*0.05]} Those antibiotics quantitated by the 4 4 4 4 3.956 4.000 3 4 1 15.0 FSIS Bioassay MRM Amikacin (aminoglycoside) NT 3 4 2 2.012 2.012 3 2 4 5.2 Apramycin (aminoglycoside) NT 4 4 2 2.401 2.401 3 2 4 6.2 Kanamycin (aminoglycoside) NT 3 4 2 2.012 2.012 3 2 4 5.2 Spectinomycin (aminoglycoside) NA-D, M 4 4 3 3.179 3.179 3 2 4 8.2 Streptomycin (aminoglycoside) NA-D 4 4 3 3.179 3.179 3 2 4 8.2 Amoxicillin (beta-lactam) NT 3 2 2 2.012 2.012 3 4 4 8.7 Ampicillin (beta-lactam) NT 3 2 2 2.012 2.012 3 4 4 8.7 Cloxacillin (beta-lactam) NT 3 2 2 2.012 2.012 3 4 4 8.7 Hetacillin (beta-lactam) NT 2 2 2 1.624 1.624 3 4 4 7.0 Ticarcillin (beta-lactam) NT 2 2 2 1.624 1.624 3 4 4 7.0 Ceftiofur (cefalosporin) NT 3 2 3 2.596 2.596 4 2 4 7.5 Cefazolin (synthetic cefalosporin) NT 3 2 2 2.012 2.012 3 2 4 5.2 Chloramphenicol NA-N 4 2 1 1.624 1.624 4 4 4 7.5 Florfenicol (chloramphen. -
Assessment Report on Tanacetum Parthenium (L.) Schultz Bip., Herba. Draft
25 September 2019 EMA/HMPC/48716/2019 Committee on Herbal Medicinal Products (HMPC) Assessment report on Tanacetum parthenium (L.) Schultz Bip., herba Draft – Revision 1 Based on Article 16d(1), Article 16f and Article 16h of Directive 2001/83/EC (traditional use) Herbal substance(s) (binomial scientific name of the plant, including plant part) Tanacetum parthenium (L.) Schultz Bip., herba Herbal preparation Powdered herbal substance Pharmaceutical form(s) Herbal preparation in solid dosage forms for oral use First assessment Rapporteur G Calapai Peer-reviewer B Kroes Revision Rapporteur A Assisi Peer-reviewer B Kroes Note: This draft assessment report is published to support the public consultation of the draft European Union herbal monograph on Tanacetum parthenium (L.) Schultz Bip., herba. It is a working document, not yet edited, and shall be further developed after the release for consultation of the monograph. Interested parties are welcome to submit comments to the HMPC secretariat, which will be taken into consideration but no ‘overview of comments received during the public consultation’ will be prepared on comments that will be received on this assessment report. The publication of this draft assessment report has been agreed to facilitate the understanding by Interested Parties of the assessment that has been carried out so far and led to the preparation of the draft monograph. Official address Domenico Scarlattilaan 6 ● 1083 HS Amsterdam ● The Netherlands Address for visits and deliveries Refer to www.ema.europa.eu/how-to-find-us Send us a question Go to www.ema.europa.eu/contact Telephone +31 (0)88 781 6000 An agency of the European Union © European Medicines Agency, 2019. -

Skin Probiotics ACCELERATING INNOVATION
ACCELERATING INNOVATION James Madison University technologies are available for licensing through its nonprofit affiliate, James Madison Innovations, Inc. Skin Probiotics Inventors: Reid Harris and Kevin Minbiole Department: Biology and Chemistry Overview Research Funding and Source: National Science Foundation James Madison University inventors have filed a patent Years of Development: 4 years application on a helpful bacterium that could potentially be Technology Readiness: Research Development used as a therapy for human skin fungus such as athlete’s foot. Patent Status: U.S. patent pending 20110002891 The JMU inventors have developed a potential process to Contact: Mary Lou Bourne, Director of Technology Transfer deliver a helpful microbe, Janthinobacterium Lividum to the James Madison University skin using a pharmaceutically acceptable carrier. The microbe Phone: (540)568-2865 E-mail: [email protected] has been shown to suppress bacterial and fungal growth on animals and in lab demonstrations. Tech Transfer and Business Model Infections can be a problem for a wide array of hosts. For JMI is interested in identifying an existing company or example, there are a variety of infections, such as bacterial, entrepreneur interested in commercializing the technology viral and/or fungal infections, that affect a large percentage of either under an exclusive or a non-exclusive license. A the human population. Tricophyton rubrum, the fungus that small company or entrepreneur could further develop the causes athlete’s foot, is responsible for approximately 46% to technology, possibly using SBIR or STTR funding. 72% of cutaneous and nail mycoses worldwide. Onychomycosis, a common and persistent fungal infection, is Market and Competition In 2008, the U.S. -

Giardiasis Importance Giardiasis, a Gastrointestinal Disease Characterized by Acute Or Chronic Diarrhea, Is Caused by Protozoan Parasites in the Genus Giardia
Giardiasis Importance Giardiasis, a gastrointestinal disease characterized by acute or chronic diarrhea, is caused by protozoan parasites in the genus Giardia. Giardia duodenalis is the major Giardia Enteritis, species found in mammals, and the only species known to cause illness in humans. This Lambliasis, organism is carried in the intestinal tract of many animals and people, with clinical signs Beaver Fever developing in some individuals, but many others remaining asymptomatic. In addition to diarrhea, the presence of G. duodenalis can result in malabsorption; some studies have implicated this organism in decreased growth in some infected children and Last Updated: December 2012 possibly decreased productivity in young livestock. Outbreaks are occasionally reported in people, as the result of mass exposure to contaminated water or food, or direct contact with infected individuals (e.g., in child care centers). People are considered to be the most important reservoir hosts for human giardiasis. The predominant genetic types of G. duodenalis usually differ in humans and domesticated animals (livestock and pets), and zoonotic transmission is currently thought to be of minor significance in causing human illness. Nevertheless, there is evidence that certain isolates may sometimes be shared, and some genetic types of G. duodenalis (assemblages A and B) should be considered potentially zoonotic. Etiology The protozoan genus Giardia (Family Giardiidae, order Giardiida) contains at least six species that infect animals and/or humans. In most mammals, giardiasis is caused by Giardia duodenalis, which is also called G. intestinalis. Both names are in current use, although the validity of the name G. intestinalis depends on the interpretation of the International Code of Zoological Nomenclature. -

The Antimycobacterial Activity of Hypericum Perforatum Herb and the Effects of Surfactants
Utah State University DigitalCommons@USU All Graduate Theses and Dissertations Graduate Studies 8-2012 The Antimycobacterial Activity of Hypericum perforatum Herb and the Effects of Surfactants Shujie Shen Utah State University Follow this and additional works at: https://digitalcommons.usu.edu/etd Part of the Engineering Commons Recommended Citation Shen, Shujie, "The Antimycobacterial Activity of Hypericum perforatum Herb and the Effects of Surfactants" (2012). All Graduate Theses and Dissertations. 1322. https://digitalcommons.usu.edu/etd/1322 This Thesis is brought to you for free and open access by the Graduate Studies at DigitalCommons@USU. It has been accepted for inclusion in All Graduate Theses and Dissertations by an authorized administrator of DigitalCommons@USU. For more information, please contact [email protected]. i THE ANTIMYCOBACTERIAL ACTIVITY OF HYPERICUM PERFORATUM HERB AND THE EFFECTS OF SURFACTANTS by Shujie Shen A thesis submitted in partial fulfillment of the requirements for the degree of MASTER OF SCIENCE in Biological Engineering Approved: Charles D. Miller, PhD Ronald C. Sims, PhD Major Professor Committee Member Marie K. Walsh, PhD Mark R. McLellan, PhD Committee Member Vice President for Research and Dean of the School of Graduate Studies UTAH STATE UNIVERSITY Logan, Utah 2012 ii Copyright © Shujie Shen 2012 All Rights Reserved iii ABSTRACT The Antimycobacterial Activity of Hypericum perforatum Herb and the Effects of Surfactants by Shujie Shen, Master of Science Utah State University, 2012 Major Professor: Dr. Charles D. Miller Department: Biological Engineering Due to the essential demands for novel anti-tuberculosis treatments for global tuberculosis control, this research investigated the antimycobacterial activity of Hypericum perforatum herb (commonly known as St. -

Health Information for International Travel 1996-97
CDCCENTERS FOR DISEASE CONTROL AND PREVENTION Health Information for International Travel 1996-97 U.S. DEPARTMENT OF HEALTH AND HUMAN SERVICES Public Health Service This document was created with FrameMaker 4.0.4 ATTENTION READERS It is impossible for an annual publication on international travel to remain absolutely current given the nature of disease transmission in the world today. For readers of this text to be the most up-to-date on travel-related diseases and recommendations, this text must be used in conjunction with the other services provided by the Travelers’ Health Section of the Centers for Disease Control and Prevention (CDC). Changes such as vaccine requirements, disease outbreaks, drug availability, or emerging infections will be posted promptly on these services. For these and other changes, please consult either our Voice or Fax Information Service at 404-332-4559 or our Internet address on the World Wide Web Server at http://www.cdc.gov or the File Transfer Protocol server at ftp.cdc.gov . Because certain countries require vaccination against yellow fever only if a traveler arrives from a country currently infected with this disease, it is essential that up-to-date information regarding infected areas be maintained for reference. The CDC publishes a biweekly "Summary of Health Information for Interna- tional Travel" (Blue Sheet) which lists yellow fever infected areas. Subscriptions to the Blue Sheet are available to health departments, physicians, travel agencies, international airlines, shipping companies, travel clinics, and other private and public agencies that advise international travelers concerning health risks they may encounter when visiting other countries. -

Revised Use-Function Classification (2007)
INTERNATIONAL PROGRAMME ON CHEMICAL SAFETY IPCS INTOX Data Management System (INTOX DMS) Revised Use-Function Classification (2007) The Use-Function Classification is used in two places in the INTOX Data Management System: the Communication Record and the Agent/Product Record. The two records are linked: if there is an agent record for a Centre Agent that is the subject of a call, the appropriate Intended Use-Function can be selected automatically in the Communication Record. The Use-Function Classification is used when generating reports, both standard and customized, and for searching the case and agent databases. In particular, INTOX standard reports use the top level headings of the Intended Use-Functions that were selected for Centre Agents in the Communication Record (e.g. if an agent was classified as an Analgesic for Human Use in the Communication Record, it would be logged as a Pharmaceutical for Human Use in the report). The Use-Function classification is very important for ensuring harmonized data collection. In version 4.4 of the software, 5 new additions were made to the top levels of the classification provided with the system for the classification of organisms (items XIV to XVIII). This is a 'convenience' classification to facilitate searching of the Communications database. A taxonomic classification for organisms is provided within the INTOX DMS Agent Explorer. In May/June 2006 INTOX users were surveyed to find out whether they had made any changes to the Use-Function Classification. These changes were then discussed at the 4th and 5th Meetings of INTOX Users. Version 4.5 of the INTOX DMS includes the revised pesticides classification (shown in full below). -

Autophagy in Trypanosomatids
Cells 2012, 1, 346-371; doi:10.3390/cells1030346 OPEN ACCESS cells ISSN 2073-4409 www.mdpi.com/journal/cells Review Autophagy in Trypanosomatids Ana Brennand 1,†, Eva Rico 2,†,‡ and Paul A. M. Michels 1,* 1 Research Unit for Tropical Diseases, de Duve Institute, Université catholique de Louvain, Avenue Hippocrate 74, postal box B1.74.01, B-1200 Brussels, Belgium; E-Mail: [email protected] 2 Department of Biochemistry and Molecular Biology, University Campus, University of Alcalá, Alcalá de Henares, Madrid, 28871, Spain; E-Mail: [email protected] † These authors contributed equally to this work. ‡ Present Address: Centre for Immunity, Infection and Evolution, Institute of Immunology and Infection Research, School of Biological Sciences, King’s Buildings, University of Edinburgh, West Mains Road, Edinburgh EH9 3JT, UK. * Author to whom correspondence should be addressed; E-Mail: [email protected]; Tel.: +32-2-7647473; Fax: +32-2-7626853. Received: 28 June 2012; in revised form: 14 July 2012 / Accepted: 16 July 2012 / Published: 27 July 2012 Abstract: Autophagy is a ubiquitous eukaryotic process that also occurs in trypanosomatid parasites, protist organisms belonging to the supergroup Excavata, distinct from the supergroup Opistokontha that includes mammals and fungi. Half of the known yeast and mammalian AuTophaGy (ATG) proteins were detected in trypanosomatids, although with low sequence conservation. Trypanosomatids such as Trypanosoma brucei, Trypanosoma cruzi and Leishmania spp. are responsible for serious tropical diseases in humans. The parasites are transmitted by insects and, consequently, have a complicated life cycle during which they undergo dramatic morphological and metabolic transformations to adapt to the different environments. -

2019 National Library of Medicine Classification Schedules
National Library of Medicine Classification 2019 Schedule S-1 QS Human Anatomy Classify here general works on normal human anatomy. Works that treat men, women, or children separately are classed here. • Classify works on anatomy of a part of the body with the part. • Classify works on surgical anatomy in WO 101. • Classify works on artistic anatomy of human or animal in NC 760-783.8. • Classify works on anatomy of animals in QL or SF. QS 1-132 Anatomy QS 504-532 Histology QS 604-681 Embryology Anatomy Note that form numbers are also used under Histology (QS 504-539) and under Embryology (QS 604-681). QS 1 Organizations. Societies (General or not elsewhere classified) (Cutter from name of organization or society) (Table G) (Used for both monographs and serials) Includes membership lists issued serially or separately. Classify directories in QS 22. Classify annual reports, journals, etc., in W1. For academies and institutes, see QS 23-24. QS 4 General works Classify here works on regional anatomy. If written for the surgeon, classify in WO 101 Surgical anatomy. Classify material on comparative anatomy in QS 124. Collected works QS 5 By several authors QS 7 By individual authors QS 9 Addresses. Essays. Lectures QS 11 History (Table G) QS 11.1 General coverage (Not Table G) QS 13 Dictionaries. Encyclopedias QS 15 Classification. Terminology (Used for both monographs and serials) QS 16 Tables. Statistics. Surveys (Table G) (Used for both monographs and serials) QS 16.1 General coverage (Not Table G) (Used for both monographs and serials) QS 17 Atlases. -

Artemether-Lumefantrine (Six-Dose Regimen) for Treating Uncomplicated Falciparum Malaria (Review)
Artemether-lumefantrine (six-dose regimen) for treating uncomplicated falciparum malaria (Review) Omari AAA, Gamble CL, Garner P This is a reprint of a Cochrane review, prepared and maintained by The Cochrane Collaboration and published in The Cochrane Library 2009, Issue 1 http://www.thecochranelibrary.com Artemether-lumefantrine (six-dose regimen) for treating uncomplicated falciparum malaria (Review) Copyright © 2009 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd. TABLE OF CONTENTS HEADER....................................... 1 ABSTRACT ...................................... 1 PLAINLANGUAGESUMMARY . 2 BACKGROUND .................................... 2 OBJECTIVES ..................................... 3 METHODS ...................................... 3 RESULTS....................................... 5 DISCUSSION ..................................... 9 AUTHORS’CONCLUSIONS . 9 ACKNOWLEDGEMENTS . 10 REFERENCES ..................................... 10 CHARACTERISTICSOFSTUDIES . 13 DATAANDANALYSES. 20 Analysis 1.1. Comparison 1 Artemether-lumefantrine vs amodiaquine, Outcome 1 Total failure by day 28. 22 Analysis 1.2. Comparison 1 Artemether-lumefantrine vs amodiaquine, Outcome 2 Total failure by day 14. 23 Analysis 1.3. Comparison 1 Artemether-lumefantrine vs amodiaquine, Outcome 3 Gametocyte carriage on day 14. 23 Analysis 2.1. Comparison 2 Artemether-lumefantrine vs chloroquine plus sulfadoxine-pyrimethamine, Outcome 1 Total failurebyday28. ................................ 24 Analysis 2.2. Comparison 2 Artemether-lumefantrine