Copper Sulfide Nanosheets with Shape-Tunable Plasmonic Properties in the NIR
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Biofilm Adhesion on the Sulfide Mineral Bornite & Implications for Astrobiology
University of Rhode Island DigitalCommons@URI Open Access Master's Theses 2019 BIOFILM ADHESION ON THE SULFIDE MINERAL BORNITE & IMPLICATIONS FOR ASTROBIOLOGY Margaret M. Wilson University of Rhode Island, [email protected] Follow this and additional works at: https://digitalcommons.uri.edu/theses Recommended Citation Wilson, Margaret M., "BIOFILM ADHESION ON THE SULFIDE MINERAL BORNITE & IMPLICATIONS FOR ASTROBIOLOGY" (2019). Open Access Master's Theses. Paper 1517. https://digitalcommons.uri.edu/theses/1517 This Thesis is brought to you for free and open access by DigitalCommons@URI. It has been accepted for inclusion in Open Access Master's Theses by an authorized administrator of DigitalCommons@URI. For more information, please contact [email protected]. BIOFILM ADHESION ON THE SULFIDE MINERAL BORNITE & IMPLICATIONS FOR ASTROBIOLOGY BY MARGARET M. WILSON A THESIS SUBMITTED IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF MASTER OF SCIENCE IN BIOLOGICAL & ENVIRONMENTAL SCIENCE UNIVERSITY OF RHODE ISLAND 2019 MASTER OF SCIENCE IN BIOLOGICAL & ENVIRONMENTAL SCIENCE THESIS OF MARGARET M. WILSON APPROVED: Thesis Committee: Major Professor Dawn Cardace José Amador Roxanne Beinart Nasser H. Zawia DEAN OF THE GRADUATE SCHOOL UNIVERSITY OF RHODE ISLAND 2019 ABSTRACT We present research observing and documenting the model organism, Pseudomonas fluorescens (P. fluorescens), building biofilm on a natural mineral substrate composed largely of bornite (Cu5FeS4), a copper-iron sulfide mineral, with closely intergrown regions of covellite (CuS) and chalcopyrite (CuFeS2). In examining biofilm establishment on sulfide minerals, we investigate a potential habitable niche for microorganisms in extraterrestrial sites. Geochemical microenvironments on Earth and in the lab can also serve as analogs for important extraterrestrial sites, such as sheltered, subsurface microenvironments on Mars. -

Leaching of Pure Chalcocite with Reject Brine and Mno2 from Manganese Nodules
metals Article Leaching of Pure Chalcocite with Reject Brine and MnO2 from Manganese Nodules David Torres 1,2,3, Emilio Trigueros 1, Pedro Robles 4 , Williams H. Leiva 5, Ricardo I. Jeldres 5 , Pedro G. Toledo 6 and Norman Toro 1,2,3,* 1 Department of Mining and Civil Engineering, Universidad Politécnica de Cartagena, 30203 Cartagena, Spain; [email protected] (D.T.); [email protected] (E.T.) 2 Faculty of Engineering and Architecture, Universidad Arturo Prat, Almirante Juan José Latorre 2901, Antofagasta 1244260, Chile 3 Departamento de Ingeniería Metalúrgica y Minas, Universidad Católica del Norte, Antofagasta 1270709, Chile 4 Escuela de Ingeniería Química, Pontificia Universidad Católica de Valparaíso, Valparaíso 2340000, Chile; [email protected] 5 Departamento de Ingeniería Química y Procesos de Minerales, Facultad de Ingeniería, Universidad de Antofagasta, Antofagasta 1270300, Chile; [email protected] (W.H.L.); [email protected] (R.I.J.) 6 Department of Chemical Engineering and Laboratory of Surface Analysis (ASIF), Universidad de Concepción, P.O. Box 160-C, Correo 3, Concepción 4030000, Chile; [email protected] * Correspondence: [email protected]; Tel.: +56-552651021 Received: 21 September 2020; Accepted: 24 October 2020; Published: 27 October 2020 Abstract: Chalcocite (Cu2S) has the fastest kinetics of dissolution of Cu in chlorinated media of all copper sulfide minerals. Chalcocite has been identified as having economic interest due to its abundance, although the water necessary for its dissolution is scarce in many regions. In this work, the replacement of fresh water by sea water or by reject brine with high chloride content from desalination plants is analyzed. -
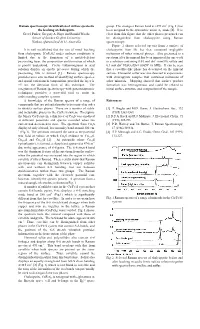
Raman Spectroscopic Identification of Surface Species in the Leaching Of
Raman spectroscopic identification of surface species in group. The strongest Raman band at »293 cm-1 (Fig. 1) has the leaching of chalcopyrite been assigned to the symmetric anion A1 mode [4]. It is Gretel Parker, Gregory A. Hope and Ronald Woods clear from this figure that the other phases presented can School of Science Griffith University be distinguished from chalcopyrite using Raman Nathan, Queensland 4111, Australia spectroscopy. Figure 2 shows selected spectra from a surface of It is well established that the rate of metal leaching chalcopyrite from Mt Isa that contained negligible from chalcopyrite [CuFeS2] under ambient conditions is inclusions of other mineral phases. Also presented is a limited due to the formation of a metal-deficient spectrum after the mineral has been immersed for one week passivating layer, the composition and formation of which in a solution containing 0.03 mol dm-3 iron(III) sulfate and -3 is poorly understood. Cyclic voltammograms in acid 0.1 mol dm H2SO4 (Eh = 0.865V vs SHE). It can be seen solution display an anodic pre-wave during which the that a covellite-like phase has developed on the mineral passivating film is formed [1]. Raman spectroscopy surface. Elemental sulfur was also detected in experiments provides an in situ method of identifying surface species, with chalcopyrite samples that contained inclusions of and spatial variations in composition, provided the layer is other minerals. Mapping showed that surface product >5 nm, the detection limit of this technique. The formation was heterogeneous and could be related to integration of Raman spectroscopy with potentiodynamic initial surface structure and composition of the sample. -

AN OCCURRENCE of the ASSEMBLAOE, NATIVE SULFTIR- COVELLITE-"Cu5 6,Fe,S6.S"", AUCANQUILCHA, CHILE Aran H. Cr-Etr&L
THE AMERICAN MINERALOGIST, VOL. 55, MAY_JUNE, 1970 AN OCCURRENCE OF THE ASSEMBLAOE, NATIVE SULFTIR- COVELLITE-"Cu5 6,Fe,S6.s"",AUCANQUILCHA, CHILE AraN H. Cr-etr<, Deportmentof GeologicalSciences, Queen's U niaersity, Kingston, Ontario. AssrnA.cr A mineral with composition near to CusFeSo has been found associated with nzrtive sulfur and covellite in the volcanic sulfur deposit at Aucanquilcha. Microprobe, optical and X-ray powder diffraction data match closell'a previously reported occurrence at Nu- kundamu, Fiji. Comparison with equilibrium synthesis by Kullerud and others indir:ates formation in the temperature range 434-482"C. INrnonucrroN Electron probe microanalysis(L6vy, 1967; Sillitoe and Clark, lt69) of naturally-occurring,supergene idaite has cast considerabledoub1. on the equation (Frenzel, 1959) of this not uncommon sulfi.de with the phaseof generalformula Cur r,Fe,Se.s,1 (Yund, 1963), which has h,een synthesizedby Merwin and Lombard (1937), Roseboomand Kullerud (1958),and Yund and Kullerud (1966).Idaite has been shown to have the compositionCurFeSa, or Cu3FeS4-,1orrd there is as yet no evidence of solid solution in nature between this and more copper-rich comF,osi- tions.The well-establishedX-ray powder data"(a:3.772 A; c:11.1U A; Yund, 1963) for hexagonal Cus.s,Fe"Se.r, are only with difficulty recon- ciled with thoseof natural idaite (Frenzel,1959, 1963), and L6vy (I\167) has suggestedthat Frenzel'soriginal powder data may be adequately fitted to a stannite-type,tetragonal cell. A differencein crystal struclure between thesephases is supported by the dissimilar reflectivity disper- sion profi.lesof natural idaite (L6vy, 1967; Sillitoe and Clark, 1969) and the synthetic "Cu5FeS6"of Merwin and l-ombard (1937; inLlvy, 1967). -

Effects of Copper on Fish and Aquatic Resources
Effects of Copper on Fish and Aquatic Resources Prepared for By Dr. Carol Ann Woody & Sarah Louise O’Neal Fisheries Research and Consulting Anchorage, Alaska June 2012 Effects of Copper on Fish and Aquatic Resources Introduction The Nushagak and Kvichak river watersheds in Bristol Bay Alaska (Figure 1) together produced over 650 million sockeye salmon during 1956-2011, about 40% of Bristol Bay production (ADFG 2012). Proposed mining of copper–sulfide ore in these watersheds will expose rocks with elevated metal concentrations including copper (Cu) (Figure 1; Cox 1996, NDM 2005a, Ghaffari et al. 2011). Because mining can increase metal concentrations in water by several orders of magnitude compared to uncontaminated sites (ATSDR 1990, USEPA 2000, Younger 2002), and because Cu can be highly toxic to aquatic life (Eisler 2000), this review focuses on risks to aquatic life from potential increased Cu inputs from proposed development. Figure 1. Map showing current mining claims (red) in Nushagak and Kvichak river watersheds as of 2011. Proven low- grade copper sulfide deposits are located in large lease block along Iliamna Lake. Documented salmon streams are outlined in dark blue. Note many regional streams have never been surveyed for salmon presence or absence. Sources: fish data from: www.adfg.alaska.gov/sf/SARR/AWC/index.cfm?ADFG =main.home mine data from Alaska Department of Natural Resources - http://www.asgdc.state.ak.us/ Core samples collected from Cu prospects near Iliamna Lake (Figure 1) show high potential for acid generation due to iron sulfides in the rock (NDM 2005a). When sulfides are exposed to oxygen and water sulfuric acid forms, which can dissolve metals in rock. -

Sediment-Hosted Copper Deposits of the World: Deposit Models and Database
Sediment-Hosted Copper Deposits of the World: Deposit Models and Database By Dennis P. Cox1, David A. Lindsey2 Donald A. Singer1, Barry C. Moring1, and Michael F. Diggles1 Including: Descriptive Model of Sediment-Hosted Cu 30b.1 by Dennis P. Cox1 Grade and Tonnage Model of Sediment-Hosted Cu by Dennis P. Cox1 and Donald A. Singer1 Descriptive Model of Reduced-Facies Cu 30b.2 By Dennis P. Cox1 Grade and Tonnage Model of Reduced Facies Cu by Dennis P. Cox1 and Donald A. Singer1 Descriptive Model of Redbed Cu 30b.3, by David A. Lindsey2 and Dennis P. Cox1 Grade and Tonnage Model of Redbed Cu by Dennis P. Cox1 and Donald A. Singer1 Descriptive Model of Revett Cu 30b.4, by Dennis P. Cox1 Grade and Tonnage Model of Revett Cu by Dennis P. Cox1 and Donald A. Singer1 Open-File Report 03-107 Version 1.3 2003, revised 2007 Available online at http://pubs.usgs.gov/of/2003/of03-107/ Any use of trade, product or firm names is for descriptive purposes only and does not imply endorsement by the U.S. Government. U.S. DEPARTMENT OF THE INTERIOR U.S. GEOLOGICAL SURVEY 1 345 Middlefield Road, Menlo Park, CA 94025 2 Box 25046, Denver Federal Center, Denver, CO 80225 Introduction This publication contains four descriptive models and four grade-tonnage models for sediment hosted copper deposits. Descriptive models are useful in exploration planning and resource assessment because they enable the user to identify deposits in the field and to identify areas on geologic and geophysical maps where deposits could occur. -

Covellite) Probed by NQR
Phase transition and anomalous electronic behavior in layered dichalcogenide CuS (covellite) probed by NQR R.R. Gainov 1*, A.V. Dooglav 1, I.N. Pen’kov 2, I.R. Mukhamedshin 1,3, N.N. Mozgova 4, A.V. Evlampiev 1, and I.A. Bryzgalov 5 1 Department of Physics, Magnetic RadioSpectroscopy Laboratory, Kazan State University, Kazan, Kremlevskaya str. 18, 420008, Russian Federation 2 Department of Geology, Kazan State University, Kazan, Kremlevskaya str. 4/5, 420111, Russian Federation 3 Laboratoire de Physique des Solides, UMR 8502, Universite Paris-Sud, 91405 Orsay, France 4 Institute of geology of ore deposits, petrography, mineralogy and geochemistry (Russian Academy of Science), Staromonetny per. 35, Moscow 109017, Russian Federation 5 Department of Geology, Moscow State University, Moscow, Vorob’evy gory, 119991, Russian Federation * Corresponding author: tel.: 7-843-2315175; fax.: 7-843-2387201. Electronic address: [email protected] (R.R. Gainov). Nuclear quadrupole resonance (NQR) on copper nuclei has been applied for studies of the electronic properties of quasi-two-dimensional low-temperature superconductor CuS (covellite) in the temperature region between 1.47 and 290 K. Two NQR signals corresponding to two non- equivalent sites of copper in the structure, Cu(1) and Cu(2), has been found. The temperature dependences of copper quadrupole frequencies, line-widths and spin-lattice relaxation rates, which so far had never been investigated so precisely for this material, altogether demonstrate the structural phase transition near 55 K, which accompanies transformations of electronic spectrum not typical for simple metals. The analysis of NQR results and their comparison with literature data show that the valence of copper ions at both sites is intermediate in character between monovalent and divalent states with the dominant of the former. -

Hydrothermal Synthesis of Copper Sulfide Flowers and Nanorods For
Research Article iMedPub Journals 2018 www.imedpub.com Journal of Nanoscience & Nanotechnology Research Vol.2 No.2:7 Hydrothermal Synthesis of Copper Sulfide Shurui Yang1, Yan Ran1, Huimin Wu1, Flowers and Nanorods for Lithium-Ion Shiquan Wang1*, Battery Applications Chuanqi Feng1 and Guohua Li2 Abstract 1 Ministry-of-Education Key Laboratory for Synthesis and Applications of Copper sulfide flowers and nanorods were prepared by a surfactant-assisted Organic Functional Molecules, Hubei hydrothermal process, using copper chloride and thiourea or thioacetamide Collaborative Innovation Center for as reactant in the presence of polyethylene glycol (PEG). The influences of Advanced Organic, Chemical Materials, sulfurating reagents and reaction temperatures on the morphologies of CuS Hubei University, Wuhan, P.R. China particles are investigated. The microstructures and morphologies of as-prepared 2 School of Chemical Engineering and CuS were characterized by using powder X-ray diffraction (XRD), scanning Materials Science, Zhejiang University of electron microscopy (SEM), transmission electron microscopy (TEM), and energy- Technology, Hangzhou, P.R. China dispersive X-ray analysis (EDX), respectively. The morphologies of CuS prepared with thiourea are mainly flower-like, but the morphologies of CuS prepared with thioacetamide are nanorods and microtubes under the same reaction conditions. Due to the stability of structure, the CuS prepared with thiourea presents better *Corresponding author: cyclability compared to the CuS prepared with thioacetamide. -

Governs the Making of Photocopies Or Other Reproductions of Copyrighted Materials
Warning Concerning Copyright Restrictions The Copyright Law of the United States (Title 17, United States Code) governs the making of photocopies or other reproductions of copyrighted materials. Under certain conditions specified in the law, libraries and archives are authorized to furnish a photocopy or other reproduction. One of these specified conditions is that the photocopy or reproduction is not to be used for any purpose other than private study, scholarship, or research. If electronic transmission of reserve material is used for purposes in excess of what constitutes "fair use," that user may be liable for copyright infringement. (Photo: Kennecott) Bingham Canyon Landslide: Analysis and Mitigation GE 487: Geological Engineering Design Spring 2015 Jake Ward 1 Honors Undergraduate Thesis Signatures: 2 Abstract On April 10, 2013, a major landslide happened at Bingham Canyon Mine near Salt Lake City, Utah. The Manefay Slide has been called the largest non-volcanic landslide in modern North American history, as it is estimated it displaced more than 145 million tons of material. No injuries or loss of life were recorded during the incident; however, the loss of valuable operating time has a number of slope stability experts wondering how to prevent future large-scale slope failure in open pit mines. This comprehensive study concerns the analysis of the landslide at Bingham Canyon Mine and the mitigation of future, large- scale slope failures. The Manefay Slide was modeled into a two- dimensional, limit equilibrium analysis program to find the controlling factors behind the slope failure. It was determined the Manefay Slide was a result of movement along a saturated, bedding plane with centralized argillic alteration. -

149. Crystal Structure of Enargite (Cu3ass4)
524 [Vol. 9, 149. Crystal Structure of Enargite (Cu3AsS4). By Katsutoshi TAKANE. Institute of Mineralogy, Petrology and Economic Geology, Tohoku Imperial University, Sendai. (Rec. Nov. 11, 1933. Comm. by S. Kozu, M.I.A., Nov. 13, 1933.) Recently, the crystal structures of copper sulphides such as covellite (CuS), wolfsbergite (CuSbS2), emplectite (CuBiS2), chalcopyrite (CuFeS2) and sulvanite (Cu3VS4), have been worked out by different authors. Among these minerals, sulvanite has been grouped in the mineral family to which enargite belongs, because of the similarity in their chemical compositions. However they are different in crystallographic nature, as sulvanite belongs the cubic system of the space group T1d, determined by Pauling and Hultgren, and enargite belongs to the orthor hombic system of the space group V12h,determined by the present author. Symmetry:-According to the morphological studies already made, enargite belongs to the orthorhombic holodedral class, the axial ratio being given as a : b : c=0.8694 : 1 : 0.8308 by Groth and Mieleitner. The Laue photograph taken from (001) shows no objection to taking the crystal as possessing the symmetry of the orthorhombic holodedral class. It is noteworthy that the photograph indicates a pseudohexagonal symmetry, of which a brief discussion has already been written in Japanese. Unit cell:-From three reflection photographs taken by rotation of three mineral rods parallel to [001], [010] and [100] respectively, immersing in the beam of the CuK ray, the distances of the layer lines were measured, and the results are The axial ratio obtained from the above figures is a : b : c=1.7341.7 1.674, which are double the values of a and c given by the goniometric method. -

Analysis of the Oxidation of Chalcopyrite,Chalcocite, Galena
PLEASE 00 Nor REMOVE FRCM LIBRARY Bureau of Mines Report of Investigations/1987 Analysis of the Oxidation of Chalcopyrite, Chalcocite, Galena, Pyrrhotite, Marcasite, and Arsenopyrite By G. W. Reimers and K. E. Hjelmstad UBRARY ""OKANERESEARC H CENTER .... RECEIVED OCT 021 1981 US BUREAU Of MINES t 315 MONTGOMBlY ",'IE. SPOICANl,. WI>. 99201 UNITED STATES DEPARTMENT OF THE INTERIOR Report of Investigations 9118 Analysis of the Oxidation of Chalcopyrite, Chalcocite, Galena!' Pyrrhotite, Marcasite, and Arsenopyrite By G. W. Reimers and K. E. Hjelmstad UNITED STATES DEPARTMENT OF THE INTERIOR Donald Paul Hodel, Secretary BUREAU OF MINES David S. Brown, Acting Director Library of Congress Cataloging in Publication Data : Reimers, G. W. (George W.) Analysis of the oxidation of chalcopyrite, chalcocite, galena, pyr rhotite, marcasite, and arsenopyrite. (Bureau of Mines report of investigations; 9118) Bibliography: p. 16 . Supt. of Docs. no.: 128.23: 9118. 1. Pyrites. 2. Oxidation. I. Hjelmstad, Kenneth E. II . Title. III. Series: Reportofinvestiga tions (United States. Bureau of Mines) ; 9118. TN23.U43 [QE389.2] 622 s [622'.8] 87-600136 CONTENTS Abst 1 Introduction ••••••••• 2 Equipment and test • .,.,.,.,.,.,., .•. .,., ..... .,.,.,.,., ... ., . ., ........ ., .... ., ..... ., .. ., . .,. 3 Samp Ie ., .. ., ., ., ., ., ., ., ., ., ., ., .. ., .• ., .. ., ..... ., •.••• ., . ., ., ..•. ., ., ., . ., ., ., ., • ., ., ., ., • 4 Results and discussion .. ., . .,.,.,. ........ .,., ...... ., ., .... " . ., . .,.,., . .,., . .,., ,.,.., ., . ., -

Minerals Found in Michigan Listed by County
Michigan Minerals Listed by Mineral Name Based on MI DEQ GSD Bulletin 6 “Mineralogy of Michigan” Actinolite, Dickinson, Gogebic, Gratiot, and Anthonyite, Houghton County Marquette counties Anthophyllite, Dickinson, and Marquette counties Aegirinaugite, Marquette County Antigorite, Dickinson, and Marquette counties Aegirine, Marquette County Apatite, Baraga, Dickinson, Houghton, Iron, Albite, Dickinson, Gratiot, Houghton, Keweenaw, Kalkaska, Keweenaw, Marquette, and Monroe and Marquette counties counties Algodonite, Baraga, Houghton, Keweenaw, and Aphrosiderite, Gogebic, Iron, and Marquette Ontonagon counties counties Allanite, Gogebic, Iron, and Marquette counties Apophyllite, Houghton, and Keweenaw counties Almandite, Dickinson, Keweenaw, and Marquette Aragonite, Gogebic, Iron, Jackson, Marquette, and counties Monroe counties Alunite, Iron County Arsenopyrite, Marquette, and Menominee counties Analcite, Houghton, Keweenaw, and Ontonagon counties Atacamite, Houghton, Keweenaw, and Ontonagon counties Anatase, Gratiot, Houghton, Keweenaw, Marquette, and Ontonagon counties Augite, Dickinson, Genesee, Gratiot, Houghton, Iron, Keweenaw, Marquette, and Ontonagon counties Andalusite, Iron, and Marquette counties Awarurite, Marquette County Andesine, Keweenaw County Axinite, Gogebic, and Marquette counties Andradite, Dickinson County Azurite, Dickinson, Keweenaw, Marquette, and Anglesite, Marquette County Ontonagon counties Anhydrite, Bay, Berrien, Gratiot, Houghton, Babingtonite, Keweenaw County Isabella, Kalamazoo, Kent, Keweenaw, Macomb, Manistee,