12. Supergene Ore and Gangue Characteristics
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Abstract in PDF Format
PGM Associations in Copper-Rich Sulphide Ore of the Oktyabr Deposit, Talnakh Deposit Group, Russia Olga A. Yakovleva1, Sergey M. Kozyrev1 and Oleg I. Oleshkevich2 1Institute Gipronickel JS, St. Petersburg, Russia 2Mining and Metallurgical Company “Noril’sk Nickel” JS, Noril’sk, Russia e-mail: [email protected] The PGM assemblages of the Cu-rich 6%; the Ni content ranges 0.8 to 1.3%, and the ratio sulphide ores occurring in the western exocontact Cu/S = 0.1-0.2. zone of the Talnakh intrusion and the Kharaelakh Pyrrhotite-chalcopyrite ore occurs at the massive orebody have been studied. Eleven ore top of ore horizons. The ore-mineral content ranges samples, weighing 20 to 200 kg, were processed 50 to 60%, and pyrrhotite amount is <15%. The ore using gravity and flotation-gravity techniques. As a grades 1.1 to 1.3% Ni, and the ratio Cu/S = 0.35- result, the gravity concentrates were obtained from 0.7. the ores and flotation products. In the gravity Chalcopyrite ore occurs at the top and on concentrates, more than 20,000 PGM grains were the flanks of the orebodies. The concentration of found and identified, using light microscopy and sulphides ranges 50 to 60%, and pyrrhotite amount EPMA. The textural and chemical characteristics of is <1%; the Ni content ranges 1.3 to 3.4%, and the PGM were documented, as well as the PGM ratio Cu/S = 0.8-0.9. distribution in different size fractions. Also, the The ore types are distinctly distinguished balance of Pt, Pd and Au distribution in ores and by the PGE content which directly depends on the process products and the PGM mass portions in chalcopyrite quantity, but not on the total sulphide various ore types were calculated. -

Biofilm Adhesion on the Sulfide Mineral Bornite & Implications for Astrobiology
University of Rhode Island DigitalCommons@URI Open Access Master's Theses 2019 BIOFILM ADHESION ON THE SULFIDE MINERAL BORNITE & IMPLICATIONS FOR ASTROBIOLOGY Margaret M. Wilson University of Rhode Island, [email protected] Follow this and additional works at: https://digitalcommons.uri.edu/theses Recommended Citation Wilson, Margaret M., "BIOFILM ADHESION ON THE SULFIDE MINERAL BORNITE & IMPLICATIONS FOR ASTROBIOLOGY" (2019). Open Access Master's Theses. Paper 1517. https://digitalcommons.uri.edu/theses/1517 This Thesis is brought to you for free and open access by DigitalCommons@URI. It has been accepted for inclusion in Open Access Master's Theses by an authorized administrator of DigitalCommons@URI. For more information, please contact [email protected]. BIOFILM ADHESION ON THE SULFIDE MINERAL BORNITE & IMPLICATIONS FOR ASTROBIOLOGY BY MARGARET M. WILSON A THESIS SUBMITTED IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF MASTER OF SCIENCE IN BIOLOGICAL & ENVIRONMENTAL SCIENCE UNIVERSITY OF RHODE ISLAND 2019 MASTER OF SCIENCE IN BIOLOGICAL & ENVIRONMENTAL SCIENCE THESIS OF MARGARET M. WILSON APPROVED: Thesis Committee: Major Professor Dawn Cardace José Amador Roxanne Beinart Nasser H. Zawia DEAN OF THE GRADUATE SCHOOL UNIVERSITY OF RHODE ISLAND 2019 ABSTRACT We present research observing and documenting the model organism, Pseudomonas fluorescens (P. fluorescens), building biofilm on a natural mineral substrate composed largely of bornite (Cu5FeS4), a copper-iron sulfide mineral, with closely intergrown regions of covellite (CuS) and chalcopyrite (CuFeS2). In examining biofilm establishment on sulfide minerals, we investigate a potential habitable niche for microorganisms in extraterrestrial sites. Geochemical microenvironments on Earth and in the lab can also serve as analogs for important extraterrestrial sites, such as sheltered, subsurface microenvironments on Mars. -

LOW TEMPERATURE HYDROTHERMAL COPPER, NICKEL, and COBALT ARSENIDE and SULFIDE ORE FORMATION Nicholas Allin
Montana Tech Library Digital Commons @ Montana Tech Graduate Theses & Non-Theses Student Scholarship Spring 2019 EXPERIMENTAL INVESTIGATION OF THE THERMOCHEMICAL REDUCTION OF ARSENITE AND SULFATE: LOW TEMPERATURE HYDROTHERMAL COPPER, NICKEL, AND COBALT ARSENIDE AND SULFIDE ORE FORMATION Nicholas Allin Follow this and additional works at: https://digitalcommons.mtech.edu/grad_rsch Part of the Geotechnical Engineering Commons EXPERIMENTAL INVESTIGATION OF THE THERMOCHEMICAL REDUCTION OF ARSENITE AND SULFATE: LOW TEMPERATURE HYDROTHERMAL COPPER, NICKEL, AND COBALT ARSENIDE AND SULFIDE ORE FORMATION by Nicholas C. Allin A thesis submitted in partial fulfillment of the requirements for the degree of Masters in Geoscience: Geology Option Montana Technological University 2019 ii Abstract Experiments were conducted to determine the relative rates of reduction of aqueous sulfate and aqueous arsenite (As(OH)3,aq) using foils of copper, nickel, or cobalt as the reductant, at temperatures of 150ºC to 300ºC. At the highest temperature of 300°C, very limited sulfate reduction was observed with cobalt foil, but sulfate was reduced to sulfide by copper foil (precipitation of Cu2S (chalcocite)) and partly reduced by nickel foil (precipitation of NiS2 (vaesite) + NiSO4·xH2O). In the 300ºC arsenite reduction experiments, Cu3As (domeykite), Ni5As2, or CoAs (langisite) formed. In experiments where both sulfate and arsenite were present, some produced minerals were sulfarsenides, which contained both sulfide and arsenide, i.e. cobaltite (CoAsS). These experiments also produced large (~10 µm along longest axis) euhedral crystals of metal-sulfide that were either imbedded or grown upon a matrix of fine-grained metal-arsenides, or, in the case of cobalt, metal-sulfarsenide. Some experimental results did not show clear mineral formation, but instead demonstrated metal-arsenic alloying at the foil edges. -

Washington State Minerals Checklist
Division of Geology and Earth Resources MS 47007; Olympia, WA 98504-7007 Washington State 360-902-1450; 360-902-1785 fax E-mail: [email protected] Website: http://www.dnr.wa.gov/geology Minerals Checklist Note: Mineral names in parentheses are the preferred species names. Compiled by Raymond Lasmanis o Acanthite o Arsenopalladinite o Bustamite o Clinohumite o Enstatite o Harmotome o Actinolite o Arsenopyrite o Bytownite o Clinoptilolite o Epidesmine (Stilbite) o Hastingsite o Adularia o Arsenosulvanite (Plagioclase) o Clinozoisite o Epidote o Hausmannite (Orthoclase) o Arsenpolybasite o Cairngorm (Quartz) o Cobaltite o Epistilbite o Hedenbergite o Aegirine o Astrophyllite o Calamine o Cochromite o Epsomite o Hedleyite o Aenigmatite o Atacamite (Hemimorphite) o Coffinite o Erionite o Hematite o Aeschynite o Atokite o Calaverite o Columbite o Erythrite o Hemimorphite o Agardite-Y o Augite o Calciohilairite (Ferrocolumbite) o Euchroite o Hercynite o Agate (Quartz) o Aurostibite o Calcite, see also o Conichalcite o Euxenite o Hessite o Aguilarite o Austinite Manganocalcite o Connellite o Euxenite-Y o Heulandite o Aktashite o Onyx o Copiapite o o Autunite o Fairchildite Hexahydrite o Alabandite o Caledonite o Copper o o Awaruite o Famatinite Hibschite o Albite o Cancrinite o Copper-zinc o o Axinite group o Fayalite Hillebrandite o Algodonite o Carnelian (Quartz) o Coquandite o o Azurite o Feldspar group Hisingerite o Allanite o Cassiterite o Cordierite o o Barite o Ferberite Hongshiite o Allanite-Ce o Catapleiite o Corrensite o o Bastnäsite -

Selective Flotation of Enargite from Copper Sulphides in Complex Ore Systems
Selective flotation of enargite from copper sulphides in complex ore systems Maedeh Tayebi-Khorami M.Sc. (Mineral Processing Engineering) B.Sc. (Mining Engineering) A thesis submitted for the degree of Doctor of Philosophy at The University of Queensland in November 2016 Sustainable Minerals Institute Abstract Recent research has demonstrated promising results showing the possibility of separating arsenic- copper sulphides from other copper minerals by controlling the potential of the flotation pulp. Most of these studies were conducted on single mineral systems, and the selective removal of arsenic- copper minerals in real ore systems is not well understood. In real ore systems, the efficiency of the separation strongly depends on the mineralogical characteristics of the ore samples. This study seeks to understand the effect of ore mineralogy on the floatability of enargite in a complex ore system, under a controlled potential flotation environment. A composite of several high arsenic-containing drill core intersections for the high arsenic sample (HAS) and a composite of some low arsenic-containing drill core intersections for the low arsenic sample (LAS) were selected from the Tampakan copper-gold deposit in the Philippines, providing a range of arsenic levels. Arsenic in the HAS sample (enargite) was practically twice that for the LAS sample. The non-enargite copper minerals (NECu) were mostly chalcopyrite and bornite in both samples. Comprehensive size-by-size, chemical and mineralogical analyses were performed on both ore samples. It was observed that the two ore samples had similar mineralogical characteristics in terms of mineral content and liberation distribution, however there are some differences in the proportions of minerals. -

Mineral Processing
Mineral Processing Foundations of theory and practice of minerallurgy 1st English edition JAN DRZYMALA, C. Eng., Ph.D., D.Sc. Member of the Polish Mineral Processing Society Wroclaw University of Technology 2007 Translation: J. Drzymala, A. Swatek Reviewer: A. Luszczkiewicz Published as supplied by the author ©Copyright by Jan Drzymala, Wroclaw 2007 Computer typesetting: Danuta Szyszka Cover design: Danuta Szyszka Cover photo: Sebastian Bożek Oficyna Wydawnicza Politechniki Wrocławskiej Wybrzeze Wyspianskiego 27 50-370 Wroclaw Any part of this publication can be used in any form by any means provided that the usage is acknowledged by the citation: Drzymala, J., Mineral Processing, Foundations of theory and practice of minerallurgy, Oficyna Wydawnicza PWr., 2007, www.ig.pwr.wroc.pl/minproc ISBN 978-83-7493-362-9 Contents Introduction ....................................................................................................................9 Part I Introduction to mineral processing .....................................................................13 1. From the Big Bang to mineral processing................................................................14 1.1. The formation of matter ...................................................................................14 1.2. Elementary particles.........................................................................................16 1.3. Molecules .........................................................................................................18 1.4. Solids................................................................................................................19 -

Key to Rocks & Minerals Collections
STATE OF MICHIGAN MINERALS DEPARTMENT OF NATURAL RESOURCES GEOLOGICAL SURVEY DIVISION A mineral is a rock substance occurring in nature that has a definite chemical composition, crystal form, and KEY TO ROCKS & MINERALS COLLECTIONS other distinct physical properties. A few of the minerals, such as gold and silver, occur as "free" elements, but by most minerals are chemical combinations of two or Harry O. Sorensen several elements just as plants and animals are Reprinted 1968 chemical combinations. Nearly all of the 90 or more Lansing, Michigan known elements are found in the earth's crust, but only 8 are present in proportions greater than one percent. In order of abundance the 8 most important elements Contents are: INTRODUCTION............................................................... 1 Percent composition Element Symbol MINERALS........................................................................ 1 of the earth’s crust ROCKS ............................................................................. 1 Oxygen O 46.46 IGNEOUS ROCKS ........................................................ 2 Silicon Si 27.61 SEDIMENTARY ROCKS............................................... 2 Aluminum Al 8.07 METAMORPHIC ROCKS.............................................. 2 Iron Fe 5.06 IDENTIFICATION ............................................................. 2 Calcium Ca 3.64 COLOR AND STREAK.................................................. 2 Sodium Na 2.75 LUSTER......................................................................... 2 Potassium -

PHASE CHANGES in Cu 2 S AS a FUNCTION of TEMPERATURE 1. PREVIOUS WORK
National Bureau of Standards Special Publication 364, Solid State Chemistry, Proceedings of 5th Materials Research Symposium, issued July 1972. PHASE CHANGES IN cu2s AS A FUNCTION OF TEMPERATURE William R. Cook, Jr. Gould Inc., Gould Laboratories Cleveland, Ohio 44108 and Case Western Reserve University Cleveland, Ohio 44106 The high-'copper phase boundary of Cu2s deviates from stoichiometry above 300 °C, first becoming copper deficient, then above - 1075 °C becoming copper rich. The maximum copper content occurs at the monotectic temperature of 1104 °C. The strong effect of oxygen on the hexagonal-cubic transition in Cu2S was confirmed; the transition was also found to be sensi tive to the type of pretreatment of the material. The high temperature tetragonal "Cu1 96s" phase is stable between Cu1.95S and Cu2s, at temperatures of - 90° to - 140 °C. The tr~nsi tion to the tetragonal phase is extremely sluggish. The true composition of djurleite has been shown to be approximately Cu1.93S. The phases near the chalcocite-digenite region of the diagram may be grouped into those with hexagonal close packing of sulfur atoms and those with cubic close packing of sulfurs. This is important in understanding rates of transformation among the various phases that occur in this area of the diagram. Key words: Chalcocite; cu2s; digenite; djurleite; nonstoichiometry; phase relations. 1. PREVIOUS WORK Fairly thorough explorations of the Cu-s phase diagram between cu2s and cu1• ,shave been made by a number of previous workers [1-8] 1 The resulting diagram may be seen in figure 1 taken largely from Roseboom [7] and Riu [8]. -

MINERALIZATION in the GOLD HILL MINING DISTRICT, TOOELE COUNTY, UTAH by H
MINERALIZATION IN THE GOLD HILL MINING DISTRICT, TOOELE COUNTY, UTAH by H. M. EI-Shatoury and J. A. Whelan UTAH GEOLOGICAL AND MINERALOGIC~4L SURVEY affiliated with THE COLLEGE OF MINES AND MINERAL INDUSTRIES University of Utah~ Salt Lake City~ Utah Bulletin 83 Price $2.25 March 1970 CONTENTS Page ABSTRACT. • • . • . • . • . • • . • . • . • • • . • • . • . • .. 5 INTRODUCTION 5 GENERAL GEOLOGY. .. 7 ECONOMIC GEOLOGY. 7 Contact Metasomatic Deposits. 11 Veins. • . 11 Quartz-Carbonate-Adularia Veins 11 Quartz Veins . 15 Calcite Veins. 15 Replacement Deposits . 15 Replacement Deposits in the Ochre Mountain Limestone 15 Replacement Deposits in the Quartz Monzonite 17 HYDROTHERMAL ALTERATION. 17 Alteration of Quartz Monzonite. • 17 Alteration of Limestones. 22 Alteration of the Manning Canyon Formation 23 Alteration of the Quartzite. 23 Alteration of Volcanic Rocks. 23 Alteration of Dike Rocks. 23 Alteration of Quartz-Carbonate Veins . 23 OXIDATION OF ORES. 23 Oxidation of the Copper-Lead-Arsenic-Zinc Replacement Deposits 24 Oxidation of Tungsten and Molybdenum Deposits. 24 Oxidation of the Lead-Zinc Deposits 25 MINERALOGY. 25 CONTROLS OF MINERAL LOCALIZATION 25 ZONAL ARRANGEMENT OF ORE DEPOSITS. 25 GENESIS OF ORE DEPOSITS. 29 DESCRIPTION OF PROPERTIES. 29 The Alvarado Mine. 29 The Cane Spring Mine 30 The Bonnemort Mine 32 The Rube Gold Mine . 32 The Frankie Mine 32 The Yellow Hammer Mine 33 The Rube Lead Mine . 34 FUTURE OF THE DISTRICT AND RECOMMENDATIONS. .. 34 ACKNOWLEDGMENTS. .. 36 REFERENCES. • . .. 36 2 ILLUSTRATIONS Page Frontis piece Figure I. Index map showing location and accessibility to the Gold Hill mining district, Utah . 4 2. Geologic map of Rodenhouse Wash area, showing occurrence of berylliferous quartz-carbonate-adularia veins and sample locations. -

Ferrilotharmeyerite Ca(Fe3+,Zn,Cu)
3+ Ferrilotharmeyerite Ca(Fe , Zn, Cu)2(AsO4)2(OH, H2O)2 c 2001-2005 Mineral Data Publishing, version 1 Crystal Data: Monoclinic. Point Group: 2/m. As subhedral crystals, to 0.6 mm, tabular on {101}, slightly elongated along [010], wedge- or lozenge-shaped, terminated by {111}, composed of multiple crystallites. Physical Properties: Cleavage: Good on {001}. Fracture: Uneven. Tenacity: Brittle. Hardness = ∼3 D(meas.) = 4.25(5) D(calc.) = 4.21–4.38 Optical Properties: Transparent to translucent. Color: Yellow, brownish yellow, yellowish brown. Streak: Very pale yellow. Luster: Adamantine to greasy. Optical Class: Biaxial (+). Pleochroism: Strong; X = olive-green or orange; Y = pale green or yellow; Z = colorless. Orientation: X = b; Y ∧ c = ∼22◦. Dispersion: r> v,distinct, inclined. Absorption: X > Y Z. α = 1.83(1) β = [1.835] γ = 1.87(1) 2V(meas.) = 40◦ Cell Data: Space Group: C2/m. a = 8.997–9.010 b = 6.236–6.246 c = 7.387–7.391 β = 115.52−115.74◦ Z=2 X-ray Powder Pattern: Tsumeb, Namibia. 3.398 (100), 3.175 (100), 2.938 (100), 2.544 (100), 4.95 (70), 2.823 (70), 2.702 (70) Chemistry: (1) (2) As2O5 48.66 48.73 Al2O3 0.13 < 0.1 Fe2O3 13.96 15.68 CuO 5.75 < 0.1 ZnO 13.94 17.88 PbO 2.13 0.14 CaO 10.86 12.07 H2O 5.85 [5.80] Total 101.28 [100.30] (1) Tsumeb, Namibia; by electron microprobe, H2O by CHN analyzer; corresponds to (Ca0.92Pb0.05)Σ=0.97(Fe0.87Zn0.81Cu0.34Al0.01)Σ=2.03(AsO4)2(OH, H2O)2. -

Gomposition and Occurrence of Electrum Atthe
L37 The Canadian M inerala g i st Vol.33,pp. 137-151(1995) GOMPOSITIONAND OCCURRENCEOF ELECTRUM ATTHE MORNINGSTAR DEPOSIT, SAN BERNARDINOCOUNTY, GALIFORNIA: EVIDENCEFOR REMOBILIZATION OF GOLD AND SILVER RONALD WYNN SIIEETS*, JAMES R. CRAIG em ROBERT J. BODNAR Depanmen of Geolngical Sciences, Virginin Polytechnic h stitate and Stale (Jniversity, 4A44 Dening Hall, Blacl<sburg, Virginin 24060, U.S-A,. Arsrnacr Elecfum, acanthiteand uytenbogaardtite have been examined from six depthswithin the tabular quartzt calcite sockwork and breccia-filled veins in the fault-zone-hostedMorning Star depositof the northeasternMojave Desert, Califomia. Six distinct types of electrum have been identified on the basis of minerat association,grain moryhology and composition. Two types, (1) p1'rite-hostedand (2) quartz-hostedelectrum, occur with acanthite after argentite and base-metalsulfide minerals in unoxidized portions of the orebody; the remaining forr types, (3) goethite-hostedelectrum, (4) electnrm cores, (5) electrumrims and (6) wire electrum,are associatedwith assemblagesof supergeneminerals in its oxidizedportions. Pyrite- hosted quartz-hostedand goethite-hostedelectrum range in compositionfrom 6l ta 75 utt.7oAu and have uniform textures and no zoning. In lower portions ofthe oxidized ore zone, electrum seemsto replacegoethite and occursas small grains on surfacesof the goethite.Textural evidencefavors supergeneremobilization of Au and Ag, which were depositedas electrum on or replacinggoethite. This type of electrumis identical in appearanceand compositionto prinary electrum,In the upper portions of the oxidized zone,secondary electum occursas a gold-rich rim on a core of elechum and as wire-like grains,both with acanthiteand uytenbogaardtite.Such secondaryelectrum contains from 78 to 93 wt./o Au. Textural relations and asso- ciated minerals suggestthat the primary electrum was hydrothermally depositedand partially remobilized by supergene processes. -
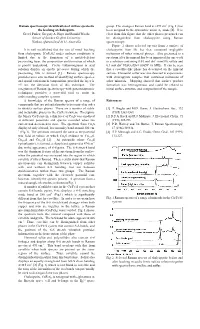
Raman Spectroscopic Identification of Surface Species in the Leaching Of
Raman spectroscopic identification of surface species in group. The strongest Raman band at »293 cm-1 (Fig. 1) has the leaching of chalcopyrite been assigned to the symmetric anion A1 mode [4]. It is Gretel Parker, Gregory A. Hope and Ronald Woods clear from this figure that the other phases presented can School of Science Griffith University be distinguished from chalcopyrite using Raman Nathan, Queensland 4111, Australia spectroscopy. Figure 2 shows selected spectra from a surface of It is well established that the rate of metal leaching chalcopyrite from Mt Isa that contained negligible from chalcopyrite [CuFeS2] under ambient conditions is inclusions of other mineral phases. Also presented is a limited due to the formation of a metal-deficient spectrum after the mineral has been immersed for one week passivating layer, the composition and formation of which in a solution containing 0.03 mol dm-3 iron(III) sulfate and -3 is poorly understood. Cyclic voltammograms in acid 0.1 mol dm H2SO4 (Eh = 0.865V vs SHE). It can be seen solution display an anodic pre-wave during which the that a covellite-like phase has developed on the mineral passivating film is formed [1]. Raman spectroscopy surface. Elemental sulfur was also detected in experiments provides an in situ method of identifying surface species, with chalcopyrite samples that contained inclusions of and spatial variations in composition, provided the layer is other minerals. Mapping showed that surface product >5 nm, the detection limit of this technique. The formation was heterogeneous and could be related to integration of Raman spectroscopy with potentiodynamic initial surface structure and composition of the sample.