AN OCCURRENCE of the ASSEMBLAOE, NATIVE SULFTIR- COVELLITE-"Cu5 6,Fe,S6.S"", AUCANQUILCHA, CHILE Aran H. Cr-Etr&L
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Biofilm Adhesion on the Sulfide Mineral Bornite & Implications for Astrobiology
University of Rhode Island DigitalCommons@URI Open Access Master's Theses 2019 BIOFILM ADHESION ON THE SULFIDE MINERAL BORNITE & IMPLICATIONS FOR ASTROBIOLOGY Margaret M. Wilson University of Rhode Island, [email protected] Follow this and additional works at: https://digitalcommons.uri.edu/theses Recommended Citation Wilson, Margaret M., "BIOFILM ADHESION ON THE SULFIDE MINERAL BORNITE & IMPLICATIONS FOR ASTROBIOLOGY" (2019). Open Access Master's Theses. Paper 1517. https://digitalcommons.uri.edu/theses/1517 This Thesis is brought to you for free and open access by DigitalCommons@URI. It has been accepted for inclusion in Open Access Master's Theses by an authorized administrator of DigitalCommons@URI. For more information, please contact [email protected]. BIOFILM ADHESION ON THE SULFIDE MINERAL BORNITE & IMPLICATIONS FOR ASTROBIOLOGY BY MARGARET M. WILSON A THESIS SUBMITTED IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF MASTER OF SCIENCE IN BIOLOGICAL & ENVIRONMENTAL SCIENCE UNIVERSITY OF RHODE ISLAND 2019 MASTER OF SCIENCE IN BIOLOGICAL & ENVIRONMENTAL SCIENCE THESIS OF MARGARET M. WILSON APPROVED: Thesis Committee: Major Professor Dawn Cardace José Amador Roxanne Beinart Nasser H. Zawia DEAN OF THE GRADUATE SCHOOL UNIVERSITY OF RHODE ISLAND 2019 ABSTRACT We present research observing and documenting the model organism, Pseudomonas fluorescens (P. fluorescens), building biofilm on a natural mineral substrate composed largely of bornite (Cu5FeS4), a copper-iron sulfide mineral, with closely intergrown regions of covellite (CuS) and chalcopyrite (CuFeS2). In examining biofilm establishment on sulfide minerals, we investigate a potential habitable niche for microorganisms in extraterrestrial sites. Geochemical microenvironments on Earth and in the lab can also serve as analogs for important extraterrestrial sites, such as sheltered, subsurface microenvironments on Mars. -

Appendix A. Supplementary Material to the Manuscript
Appendix A. Supplementary material to the manuscript: The role of crustal and eruptive processes versus source variations in controlling the oxidation state of iron in Central Andean magmas 1. Continental crust beneath the CVZ Country Rock The basement beneath the sampled portion of the CVZ belongs to the Paleozoic Arequipa- Antofalla terrain – a high temperature metamorphic terrain with abundant granitoid intrusions that formed in response to Paleozoic subduction (Lucassen et al., 2000; Ramos et al., 1986). In Northern Chile and Northwestern Argentina this Paleozoic metamorphic-magmatic basement is largely homogeneous and felsic in composition, consistent with the thick, weak, and felsic properties of the crust beneath the CVZ (Beck et al., 1996; Fig. A.1). Neodymium model ages of exposed Paleozoic metamorphic-magmatic basement and sediments suggest a uniform Proterozoic protolith, itself derived from intrusions and sedimentary rock (Lucassen et al., 2001). AFC Model Parameters Pervasive assimilation of continental crust in the Central Andean ignimbrite magmas is well established (Hildreth and Moorbath, 1988; Klerkx et al., 1977; Fig. A.1) and has been verified by detailed analysis of radiogenic isotopes (e.g. 87Sr/86Sr and 143Nd/144Nd) on specific systems within the CVZ (Kay et al., 2011; Lindsay et al., 2001; Schmitt et al., 2001; Soler et al., 2007). Isotopic results indicate that the CVZ magmas are the result of mixing between a crustal endmember, mainly gneisses and plutonics that have a characteristic crustal signature of high 87Sr/86Sr and low 145Nd/144Nd, and the asthenospheric mantle (low 87Sr/86Sr and high 145Nd/144Nd; Fig. 2). In Figure 2, we model the amount of crustal assimilation required to produce the CVZ magmas that are targeted in this study. -

Full-Text PDF (Final Published Version)
Pritchard, M. E., de Silva, S. L., Michelfelder, G., Zandt, G., McNutt, S. R., Gottsmann, J., West, M. E., Blundy, J., Christensen, D. H., Finnegan, N. J., Minaya, E., Sparks, R. S. J., Sunagua, M., Unsworth, M. J., Alvizuri, C., Comeau, M. J., del Potro, R., Díaz, D., Diez, M., ... Ward, K. M. (2018). Synthesis: PLUTONS: Investigating the relationship between pluton growth and volcanism in the Central Andes. Geosphere, 14(3), 954-982. https://doi.org/10.1130/GES01578.1 Publisher's PDF, also known as Version of record License (if available): CC BY-NC Link to published version (if available): 10.1130/GES01578.1 Link to publication record in Explore Bristol Research PDF-document This is the final published version of the article (version of record). It first appeared online via Geo Science World at https://doi.org/10.1130/GES01578.1 . Please refer to any applicable terms of use of the publisher. University of Bristol - Explore Bristol Research General rights This document is made available in accordance with publisher policies. Please cite only the published version using the reference above. Full terms of use are available: http://www.bristol.ac.uk/red/research-policy/pure/user-guides/ebr-terms/ Research Paper THEMED ISSUE: PLUTONS: Investigating the Relationship between Pluton Growth and Volcanism in the Central Andes GEOSPHERE Synthesis: PLUTONS: Investigating the relationship between pluton growth and volcanism in the Central Andes GEOSPHERE; v. 14, no. 3 M.E. Pritchard1,2, S.L. de Silva3, G. Michelfelder4, G. Zandt5, S.R. McNutt6, J. Gottsmann2, M.E. West7, J. Blundy2, D.H. -
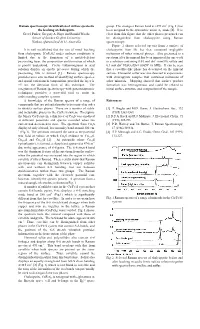
Raman Spectroscopic Identification of Surface Species in the Leaching Of
Raman spectroscopic identification of surface species in group. The strongest Raman band at »293 cm-1 (Fig. 1) has the leaching of chalcopyrite been assigned to the symmetric anion A1 mode [4]. It is Gretel Parker, Gregory A. Hope and Ronald Woods clear from this figure that the other phases presented can School of Science Griffith University be distinguished from chalcopyrite using Raman Nathan, Queensland 4111, Australia spectroscopy. Figure 2 shows selected spectra from a surface of It is well established that the rate of metal leaching chalcopyrite from Mt Isa that contained negligible from chalcopyrite [CuFeS2] under ambient conditions is inclusions of other mineral phases. Also presented is a limited due to the formation of a metal-deficient spectrum after the mineral has been immersed for one week passivating layer, the composition and formation of which in a solution containing 0.03 mol dm-3 iron(III) sulfate and -3 is poorly understood. Cyclic voltammograms in acid 0.1 mol dm H2SO4 (Eh = 0.865V vs SHE). It can be seen solution display an anodic pre-wave during which the that a covellite-like phase has developed on the mineral passivating film is formed [1]. Raman spectroscopy surface. Elemental sulfur was also detected in experiments provides an in situ method of identifying surface species, with chalcopyrite samples that contained inclusions of and spatial variations in composition, provided the layer is other minerals. Mapping showed that surface product >5 nm, the detection limit of this technique. The formation was heterogeneous and could be related to integration of Raman spectroscopy with potentiodynamic initial surface structure and composition of the sample. -

Burns Et Al 2015.Pdf
Earth and Planetary Science Letters 422 (2015) 75–86 Contents lists available at ScienceDirect Earth and Planetary Science Letters www.elsevier.com/locate/epsl Recording the transition from flare-up to steady-state arc magmatism at the Purico–Chascon volcanic complex, northern Chile ∗ Dale H. Burns a, ,1, Shanaka L. de Silva a, Frank Tepley III a, Axel K. Schmitt b, Matthew W. Loewen a a College of Earth, Ocean, and Atmospheric Sciences, Oregon State University, Corvallis, OR 97731, USA b Department of Earth, Planetary, and Space Sciences, University of California at Los Angeles, Los Angeles, CA 90095, USA a r t i c l e i n f o a b s t r a c t Article history: The long-term evolution of continental magmatic arcs is episodic, where a few transient events of high Received 6 December 2014 magmatic flux or flare-ups punctuate the low-flux magmatism or “steady state” that makes up most Received in revised form 31 March 2015 of the arc history. How this duality manifests in terms of differences in crustal architecture, magma Accepted 1 April 2015 dynamics and chemistry, and the time scale over which transitions occur is poorly known. Herein Available online 22 April 2015 we use multiscale geochemical and isotopic characteristics coupled with geothermobarometry at the Editor: T. Mather Purico–Chascon Volcanic Complex (PCVC) in the Central Andes to identify a transition from flare-up to ∼ Keywords: steady state arc magmatism over 800 kyr during which significant changes in upper crustal magmatic Central Andes dynamics are recorded. continental arc evolution The PCVC is one of the youngest volcanic centers related to a 10–1 Ma ignimbrite flare-up in the ignimbrite flare-up Altiplano–Puna Volcanic Complex of the Central Andes. -

Outlook on Climate Change Adaptation in the Tropical Andes Mountains
MOUNTAIN ADAPTATION OUTLOOK SERIES Outlook on climate change adaptation in the Tropical Andes mountains 1 Southern Bogota, Colombia photo: cover Front DISCLAIMER The development of this publication has been supported by the United Nations Environment Programme (UNEP) in the context of its inter-regional project “Climate change action in developing countries with fragile mountainous ecosystems from a sub-regional perspective”, which is financially co-supported by the Government Production Team of Austria (Austrian Federal Ministry of Agriculture, Forestry, Tina Schoolmeester, GRID-Arendal Environment and Water Management). Miguel Saravia, CONDESAN Magnus Andresen, GRID-Arendal Julio Postigo, CONDESAN, Universidad del Pacífico Alejandra Valverde, CONDESAN, Pontificia Universidad Católica del Perú Matthias Jurek, GRID-Arendal Björn Alfthan, GRID-Arendal Silvia Giada, UNEP This synthesis publication builds on the main findings and results available on projects and activities that have been conducted. Contributors It is based on available information, such as respective national Angela Soriano, CONDESAN communications by countries to the United Nations Framework Bert de Bievre, CONDESAN Convention on Climate Change (UNFCCC) and peer-reviewed Boris Orlowsky, University of Zurich, Switzerland literature. It is based on review of existing literature and not on new Clever Mafuta, GRID-Arendal scientific results generated through the project. Dirk Hoffmann, Instituto Boliviano de la Montana - BMI Edith Fernandez-Baca, UNDP The contents of this publication do not necessarily reflect the Eva Costas, Ministry of Environment, Ecuador views or policies of UNEP, contributory organizations or any Gabriela Maldonado, CONDESAN governmental authority or institution with which its authors or Harald Egerer, UNEP contributors are affiliated, nor do they imply any endorsement. -

Covellite) Probed by NQR
Phase transition and anomalous electronic behavior in layered dichalcogenide CuS (covellite) probed by NQR R.R. Gainov 1*, A.V. Dooglav 1, I.N. Pen’kov 2, I.R. Mukhamedshin 1,3, N.N. Mozgova 4, A.V. Evlampiev 1, and I.A. Bryzgalov 5 1 Department of Physics, Magnetic RadioSpectroscopy Laboratory, Kazan State University, Kazan, Kremlevskaya str. 18, 420008, Russian Federation 2 Department of Geology, Kazan State University, Kazan, Kremlevskaya str. 4/5, 420111, Russian Federation 3 Laboratoire de Physique des Solides, UMR 8502, Universite Paris-Sud, 91405 Orsay, France 4 Institute of geology of ore deposits, petrography, mineralogy and geochemistry (Russian Academy of Science), Staromonetny per. 35, Moscow 109017, Russian Federation 5 Department of Geology, Moscow State University, Moscow, Vorob’evy gory, 119991, Russian Federation * Corresponding author: tel.: 7-843-2315175; fax.: 7-843-2387201. Electronic address: [email protected] (R.R. Gainov). Nuclear quadrupole resonance (NQR) on copper nuclei has been applied for studies of the electronic properties of quasi-two-dimensional low-temperature superconductor CuS (covellite) in the temperature region between 1.47 and 290 K. Two NQR signals corresponding to two non- equivalent sites of copper in the structure, Cu(1) and Cu(2), has been found. The temperature dependences of copper quadrupole frequencies, line-widths and spin-lattice relaxation rates, which so far had never been investigated so precisely for this material, altogether demonstrate the structural phase transition near 55 K, which accompanies transformations of electronic spectrum not typical for simple metals. The analysis of NQR results and their comparison with literature data show that the valence of copper ions at both sites is intermediate in character between monovalent and divalent states with the dominant of the former. -

Volcanology and Petrology of Volcán Miño, Andean Central Volcanic Zone
AN ABSTRACT OF THE THESIS OF Claire M. McKee for the degree of Master of Science in Geology presented on June 29, 2001. Title: Volcanology and Petrology of VolcáIi Miño, Andean Central Volcanic Zone. Redacted for Privacy Anitá'L. Grunder Volcán Miño (21011'S) is located on the westernmost periphery of a long- lived complex of stratovolcarioes and domes called the Aucanquilcha Complex. The Aucanquilcha Complex ranges in age from 11 Ma to 1-lolocene and lies along the main N-S trending axis of Quaternary volcanoes in the Andean Central Volcanic Zone (CVZ). Volcán Aucanquitcha lies at the center of the complex and forms a ridge extending 10 km in an east-west direction; defined by a distinct cluster of andesite and dacite stratocones, dacite domes and a prominent collapse structure and two debris avalanche deposits. In contrast to the main edifice, Volcán Miño (5611 m) is a steep-sided, symmetric andesitic stratovolcano. Volcán Miño lavas range in age from 3.0 to 3.7 Ma and eruptive products are dominantly two-pyroxene ± hornblende andesites. Basaltic andesites and dacites are rare. - ----- - ------ ---I, ..--.- __, IuIVULJ, LII Volcán Miño lavas conform to regional med- to high-potassium caic-alkaline trends and are characterized by subduction-related light rare earth and large ion lithophile-element enrichments and high field strength element depletions. Miño lavas are distinctive in that they display a restricted range in whole-rock composition, 60±2 weight percent Si02. Despite this whole- rock compositional homogeneity, lavas are texturally and mineralogically diverse as evidenced by variations of proportions and textures of clinopyroxene, orthopyroxene, and amphibole in assemblages with similar weight percent Si02. -

Convergent Margin Magmatism in the Central Andes and Its Near Antipodes in Western Indonesia: Spatiotemporal and Geochemical Considerations
AN ABSTRACT OF THE DISSERTATION OF Morgan J. Salisbury for the degree of Doctor of Philosophy in Geology presented on June 3, 2011. Title: Convergent Margin Magmatism in the Central Andes and its Near Antipodes in Western Indonesia: Spatiotemporal and Geochemical Considerations Abstract approved: ________________________________________________________________________ Adam J.R. Kent This dissertation combines volcanological research of three convergent continental margins. Chapters 1 and 5 are general introductions and conclusions, respectively. Chapter 2 examines the spatiotemporal development of the Altiplano-Puna volcanic complex in the Lípez region of southwest Bolivia, a locus of a major Neogene ignimbrite flare- up, yet the least studied portion of the Altiplano-Puna volcanic complex of the Central Andes. New mapping and laser-fusion 40Ar/39Ar dating of sanidine and biotite from 56 locations, coupled with paleomagnetic data, refine the timing and volumes of ignimbrite emplacement in Bolivia and northern Chile to reveal that monotonous intermediate volcanism was prodigious and episodic throughout the complex. 40Ar/39Ar age determinations of 13 ignimbrites from northern Chile previously dated by the K-Ar method improve the overall temporal resolution of Altiplano-Puna volcanic complex development. Together with new and updated volume estimates, the new age determinations demonstrate a distinct onset of Altiplano-Puna volcanic complex ignimbrite volcanism with modest output rates beginning ~11 Ma, an episodic middle phase with the highest eruption rates between 8 and 3 Ma, followed by a general decline in volcanic output. The cyclic nature of individual caldera complexes and the spatiotemporal pattern of the volcanic field as a whole are consistent with both incremental construction of plutons as well as a composite Cordilleran batholith. -

149. Crystal Structure of Enargite (Cu3ass4)
524 [Vol. 9, 149. Crystal Structure of Enargite (Cu3AsS4). By Katsutoshi TAKANE. Institute of Mineralogy, Petrology and Economic Geology, Tohoku Imperial University, Sendai. (Rec. Nov. 11, 1933. Comm. by S. Kozu, M.I.A., Nov. 13, 1933.) Recently, the crystal structures of copper sulphides such as covellite (CuS), wolfsbergite (CuSbS2), emplectite (CuBiS2), chalcopyrite (CuFeS2) and sulvanite (Cu3VS4), have been worked out by different authors. Among these minerals, sulvanite has been grouped in the mineral family to which enargite belongs, because of the similarity in their chemical compositions. However they are different in crystallographic nature, as sulvanite belongs the cubic system of the space group T1d, determined by Pauling and Hultgren, and enargite belongs to the orthor hombic system of the space group V12h,determined by the present author. Symmetry:-According to the morphological studies already made, enargite belongs to the orthorhombic holodedral class, the axial ratio being given as a : b : c=0.8694 : 1 : 0.8308 by Groth and Mieleitner. The Laue photograph taken from (001) shows no objection to taking the crystal as possessing the symmetry of the orthorhombic holodedral class. It is noteworthy that the photograph indicates a pseudohexagonal symmetry, of which a brief discussion has already been written in Japanese. Unit cell:-From three reflection photographs taken by rotation of three mineral rods parallel to [001], [010] and [100] respectively, immersing in the beam of the CuK ray, the distances of the layer lines were measured, and the results are The axial ratio obtained from the above figures is a : b : c=1.7341.7 1.674, which are double the values of a and c given by the goniometric method. -

Minerals Found in Michigan Listed by County
Michigan Minerals Listed by Mineral Name Based on MI DEQ GSD Bulletin 6 “Mineralogy of Michigan” Actinolite, Dickinson, Gogebic, Gratiot, and Anthonyite, Houghton County Marquette counties Anthophyllite, Dickinson, and Marquette counties Aegirinaugite, Marquette County Antigorite, Dickinson, and Marquette counties Aegirine, Marquette County Apatite, Baraga, Dickinson, Houghton, Iron, Albite, Dickinson, Gratiot, Houghton, Keweenaw, Kalkaska, Keweenaw, Marquette, and Monroe and Marquette counties counties Algodonite, Baraga, Houghton, Keweenaw, and Aphrosiderite, Gogebic, Iron, and Marquette Ontonagon counties counties Allanite, Gogebic, Iron, and Marquette counties Apophyllite, Houghton, and Keweenaw counties Almandite, Dickinson, Keweenaw, and Marquette Aragonite, Gogebic, Iron, Jackson, Marquette, and counties Monroe counties Alunite, Iron County Arsenopyrite, Marquette, and Menominee counties Analcite, Houghton, Keweenaw, and Ontonagon counties Atacamite, Houghton, Keweenaw, and Ontonagon counties Anatase, Gratiot, Houghton, Keweenaw, Marquette, and Ontonagon counties Augite, Dickinson, Genesee, Gratiot, Houghton, Iron, Keweenaw, Marquette, and Ontonagon counties Andalusite, Iron, and Marquette counties Awarurite, Marquette County Andesine, Keweenaw County Axinite, Gogebic, and Marquette counties Andradite, Dickinson County Azurite, Dickinson, Keweenaw, Marquette, and Anglesite, Marquette County Ontonagon counties Anhydrite, Bay, Berrien, Gratiot, Houghton, Babingtonite, Keweenaw County Isabella, Kalamazoo, Kent, Keweenaw, Macomb, Manistee, -

Chalcocite Several Centimeters Wide Were Found (Butler and Burbank, 1929)
Veinlets of solid chalcocite several centimeters wide were found (Butler and Burbank, 1929). 2. CHALCOCITE Isle Royale mine: In fissures with “ankerite” and Cu2S “specularite.” Lane (1911) reports veins of Widespread, but historically not significant as an chalcocite, bornite, chalcopyrite, “whitneyite,” ore mineral in Michigan, except at the White Pine pyrrhotite(?), natrolite, analcime, and “adularia.” 3. deposit; in fissure veins cutting various rocks of Centennial mine: Disseminated in the Calumet and the native copper lodes and locally disseminated in Hecla Conglomerate. 4. Champion mine: In the lodes themselves; also present in a variety of veinlets with silver. Some as very fine-grained metalliferous veins. Twelve chalcocite deposits pulverulent material consisting of hexagonal have been located on the Keweenaw Peninsula, the platelets less than 0.5 mm across (Koenig, 1902). most notable being the Mount Bohemia and 5. Portage mine: In fissure veins with quartz and Gratiot Lake deposits (Robertson, 1975; Maki, orange calcite. 6. Wolverine mine. 7. Osceola 1999). Maki (1999) estimates the Gratiot Lake mine: As complex microcrystals and crusts on deposit may contain as much as 4.5 million metric prehnite from the No. 10 shaft (Falster, 1978). 8. tons of ore with an average grade of 2.3% copper. East slope Six Mile Hill, southwest of Houghton: Northern Peninsula. Networks of veins with copper, calcite, epidote, prehnite, datolite, and a considerable amount of chalcocite (Rominger, 1895). 9. Laurium mine: (Morris, 1983). Iron County: Sherwood and Buck iron mines: With other sulfides and uraninite (Vickers, 1956b; James et al., 1968). Figure 54: Chalcocite crystals from the White Pine mine, White Pine, Ontonagon County.