Covellite) Probed by NQR
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Paolovite Pd2sn C 2001-2005 Mineral Data Publishing, Version 1
Paolovite Pd2Sn c 2001-2005 Mineral Data Publishing, version 1 Crystal Data: Orthorhombic. Point Group: 2/m 2/m 2/m. As irregular grains embedded in other minerals. Twinning: Polysynthetic. Physical Properties: Hardness = n.d. VHN = 329–378 (10 g load). D(meas.) = n.d. D(calc.) = 11.08 Optical Properties: Opaque. Color: Lilac-rose. Luster: Metallic. Pleochroism: Dark lilac-rose to pale rose. R1–R2: (400) — , (420) 44.4–46.5, (440) 44.6–47.2, (460) 45.0–48.2, (480) 45.5–49.1, (500) 45.9–50.3, (520) 46.7–51.6, (540) 47.7–52.8, (560) 49.0–54.1, (580) 50.9–55.4, (600) 52.9–56.7, (620) 55.3–57.7, (640) 57.7–59.2, (660) 59.8–59.5, (680) 61.5–60.3, (700) 62.7–60.6 Cell Data: Space Group: P bnm. a = 8.11(1) b = 5.662(6) c = 4.324(2) Z = 4 X-ray Powder Pattern: Oktyabr deposit, Russia. 2.28 (100), 2.16 (70), 1.955 (50), 2.36 (40), 1.397 (40), 1.315 (40), 1.120 (40) Chemistry: (1) (2) (3) Pd 64.8 64.3 64.19 Pt 2.5 Sn 35.5 35.0 35.81 Sb 0.3 Bi 0.2 Total 103.3 99.3 100.00 (1) Oktyabr deposit, Russia; by electron microprobe, corresponding to (Pd1.98Pt0.04)Σ=2.02Sn0.98. (2) Western Platinum mine, South Africa; by electron microprobe, corresponding to Pd2.02Sn0.98. (3) Pd2Sn. Occurrence: In Cu–Ni sulfide ores; in cubanite–chalcopyrite, cubanite–talnakhite, and cubanite–mooihoekite assemblages (Oktyabr deposit, Russia). -

Biofilm Adhesion on the Sulfide Mineral Bornite & Implications for Astrobiology
University of Rhode Island DigitalCommons@URI Open Access Master's Theses 2019 BIOFILM ADHESION ON THE SULFIDE MINERAL BORNITE & IMPLICATIONS FOR ASTROBIOLOGY Margaret M. Wilson University of Rhode Island, [email protected] Follow this and additional works at: https://digitalcommons.uri.edu/theses Recommended Citation Wilson, Margaret M., "BIOFILM ADHESION ON THE SULFIDE MINERAL BORNITE & IMPLICATIONS FOR ASTROBIOLOGY" (2019). Open Access Master's Theses. Paper 1517. https://digitalcommons.uri.edu/theses/1517 This Thesis is brought to you for free and open access by DigitalCommons@URI. It has been accepted for inclusion in Open Access Master's Theses by an authorized administrator of DigitalCommons@URI. For more information, please contact [email protected]. BIOFILM ADHESION ON THE SULFIDE MINERAL BORNITE & IMPLICATIONS FOR ASTROBIOLOGY BY MARGARET M. WILSON A THESIS SUBMITTED IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF MASTER OF SCIENCE IN BIOLOGICAL & ENVIRONMENTAL SCIENCE UNIVERSITY OF RHODE ISLAND 2019 MASTER OF SCIENCE IN BIOLOGICAL & ENVIRONMENTAL SCIENCE THESIS OF MARGARET M. WILSON APPROVED: Thesis Committee: Major Professor Dawn Cardace José Amador Roxanne Beinart Nasser H. Zawia DEAN OF THE GRADUATE SCHOOL UNIVERSITY OF RHODE ISLAND 2019 ABSTRACT We present research observing and documenting the model organism, Pseudomonas fluorescens (P. fluorescens), building biofilm on a natural mineral substrate composed largely of bornite (Cu5FeS4), a copper-iron sulfide mineral, with closely intergrown regions of covellite (CuS) and chalcopyrite (CuFeS2). In examining biofilm establishment on sulfide minerals, we investigate a potential habitable niche for microorganisms in extraterrestrial sites. Geochemical microenvironments on Earth and in the lab can also serve as analogs for important extraterrestrial sites, such as sheltered, subsurface microenvironments on Mars. -

Cu-Fe-S Phases in Lunar Rocks
American Mineralogist, Volume 58, pages 952-954, 1973 Cu-Fe-SPhases in LunarRocks LawnnNcn A. Tevronl Departmentol Geosciences,Purdue Uniuersity, Lalaye t te, I ndiana47 907 KBNNrrn L. Wnrrnus Departmentof Applied Earth Sciences,Stanlord Uniaersity, Stanford, Calilornia 943 0 5 Abstract The sulfide minerals reported to be present in lunar rocks include troilite, mackinawite, the discredited mineral "chalcopyrrhotite", sphalerite, chalcopyrite, and cubanite. Apollo 12 sam- ple l2D2l,l34 contains these last two minerals, and the first chemical analyses of the Cu-Fe-S phases are presented. These minerals occur along cracks and grain boundaries within troilite and are probably exsolution products formed at low temperatures (i.e, 100"-300'C) from a cupriferous troilite. Introduction Cu-Fe-S Minerals The opaqueminerals, although constituting only a "Chalcopyrrhotite", a discreditedmineral (Yund minor amount of a given sample,have proven useful and Kullerud, 1966; Cabri, 7967) , hasbeen reported as indicators of the genesisand cooling histories of from Apollo 12, 14, and 15 rocks (El Goresyet al, the lunar rocks (e.9., Reid, 1971; Taylor and Mc- l97la,b; 1972a,b). It wasdescribed as a yellowish, Callister, 19721,Taylor et al, I973b). The most chalcopyrite-coloredphase which appearsisotropic abundant opaque mineral is ilmenite, and many of and is alwaysfound within troilite. It is probablethat the oxide phasesin lunar rocks are unique (e.6'., this phase is either talnakhite, a mineral originally armalcolite as per Anderson et al, l97A). In addi- describedby Cabri (1967) andhaving a composition tion to native Fe metal, native Cu, and schreibersite, of CusFesSro(Cabri and Harris, 1971) near chal- the remaining opaqueminerals consistof sulfides. -

MACKINAWITE from SOUTH AFRICA W. C J. Van RDNSBU*.O, Geological Surtey Oj Soulh Africa, Preloria, Soulh A.[Ri Co, L. Lrbnunsbyc
TI.IE AMERICAN MINERALOGIST, VOL 52, JULY AUGUST, 1967 MACKINAWITE FROM SOUTH AFRICA W. C J. vAN RDNSBU*.o,Geological Surtey oJ Soulh Africa, Preloria,Soulh A.[rico, AND L. LrBnuNsByc, Deparlment of Geology,Llnit:ersity of Pretoria, Pre!oria,Soul h Africa. ABSTRAcT Mackinawite has been identified in mafic and ultramafic rocks of the Bushveld igneous complex and Insizwa and also in the carbonatite and pegmatoid of Loolekop, Phalaborrva complex in South Africa. The mineral occurs predominantly as somewhat irregular to oriented intergrowths in pentlandite in all these occurrences and less commonly as regular oriented lamellae in chalcopyrite and rarely in cubanite. The textural evidence suggests that mackinawite may represent an exsolution product of pentlandite, chalcopyrite and cubanite. INrnonucrroN The iron sulphide, mackinawite was recently named in a paper by Evans, et al, (1964). Natural occurrencesof this mineral f rom Finland were describedby- Kouvo et al (1963)and from the Muskox intrusion in Canada by Chamberlain and Delabio (1965). During the presentinvestigation mackinawite was distinguishedfrom valleriite in the carbonatite and pegmatoid of Loolekop, Phalaborwa complex, in the Bushveld igneous complex, and from Insizwa, Cape Province.The mode of occurrenceof the mackinawite in the carbonatite difiers somewhatfrom that in the mafic rocks of Insizwa and the Bush- veld complex. MrNnn.q.rocv The physicaland optical propertiesof mackinawitefrom the Bushveid complex,Insizwa and Loolekop are very similar and are in closeagree- ment with the propertiesgiven for the same mineral from the Muskox intrusion by Chamberlain and Delabio (1965). The mineral takes a fairly good polish,particularly after buffing with a chromic oxide slurry. -
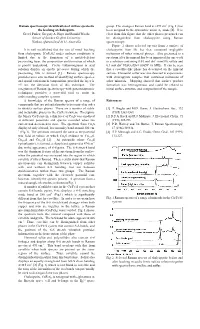
Raman Spectroscopic Identification of Surface Species in the Leaching Of
Raman spectroscopic identification of surface species in group. The strongest Raman band at »293 cm-1 (Fig. 1) has the leaching of chalcopyrite been assigned to the symmetric anion A1 mode [4]. It is Gretel Parker, Gregory A. Hope and Ronald Woods clear from this figure that the other phases presented can School of Science Griffith University be distinguished from chalcopyrite using Raman Nathan, Queensland 4111, Australia spectroscopy. Figure 2 shows selected spectra from a surface of It is well established that the rate of metal leaching chalcopyrite from Mt Isa that contained negligible from chalcopyrite [CuFeS2] under ambient conditions is inclusions of other mineral phases. Also presented is a limited due to the formation of a metal-deficient spectrum after the mineral has been immersed for one week passivating layer, the composition and formation of which in a solution containing 0.03 mol dm-3 iron(III) sulfate and -3 is poorly understood. Cyclic voltammograms in acid 0.1 mol dm H2SO4 (Eh = 0.865V vs SHE). It can be seen solution display an anodic pre-wave during which the that a covellite-like phase has developed on the mineral passivating film is formed [1]. Raman spectroscopy surface. Elemental sulfur was also detected in experiments provides an in situ method of identifying surface species, with chalcopyrite samples that contained inclusions of and spatial variations in composition, provided the layer is other minerals. Mapping showed that surface product >5 nm, the detection limit of this technique. The formation was heterogeneous and could be related to integration of Raman spectroscopy with potentiodynamic initial surface structure and composition of the sample. -

AN OCCURRENCE of the ASSEMBLAOE, NATIVE SULFTIR- COVELLITE-"Cu5 6,Fe,S6.S"", AUCANQUILCHA, CHILE Aran H. Cr-Etr&L
THE AMERICAN MINERALOGIST, VOL. 55, MAY_JUNE, 1970 AN OCCURRENCE OF THE ASSEMBLAOE, NATIVE SULFTIR- COVELLITE-"Cu5 6,Fe,S6.s"",AUCANQUILCHA, CHILE AraN H. Cr-etr<, Deportmentof GeologicalSciences, Queen's U niaersity, Kingston, Ontario. AssrnA.cr A mineral with composition near to CusFeSo has been found associated with nzrtive sulfur and covellite in the volcanic sulfur deposit at Aucanquilcha. Microprobe, optical and X-ray powder diffraction data match closell'a previously reported occurrence at Nu- kundamu, Fiji. Comparison with equilibrium synthesis by Kullerud and others indir:ates formation in the temperature range 434-482"C. INrnonucrroN Electron probe microanalysis(L6vy, 1967; Sillitoe and Clark, lt69) of naturally-occurring,supergene idaite has cast considerabledoub1. on the equation (Frenzel, 1959) of this not uncommon sulfi.de with the phaseof generalformula Cur r,Fe,Se.s,1 (Yund, 1963), which has h,een synthesizedby Merwin and Lombard (1937), Roseboomand Kullerud (1958),and Yund and Kullerud (1966).Idaite has been shown to have the compositionCurFeSa, or Cu3FeS4-,1orrd there is as yet no evidence of solid solution in nature between this and more copper-rich comF,osi- tions.The well-establishedX-ray powder data"(a:3.772 A; c:11.1U A; Yund, 1963) for hexagonal Cus.s,Fe"Se.r, are only with difficulty recon- ciled with thoseof natural idaite (Frenzel,1959, 1963), and L6vy (I\167) has suggestedthat Frenzel'soriginal powder data may be adequately fitted to a stannite-type,tetragonal cell. A differencein crystal struclure between thesephases is supported by the dissimilar reflectivity disper- sion profi.lesof natural idaite (L6vy, 1967; Sillitoe and Clark, 1969) and the synthetic "Cu5FeS6"of Merwin and l-ombard (1937; inLlvy, 1967). -

High-Pressure Crystal Chemistry of Cubanite, Cufers
American Mineralogist, Volume 77, pages 937-944, 1992 High-pressure crystal chemistry of cubanite,CuFerS, CarnnnrNr McClvrmoNr* JrNvrrN ZruNG, RonBnr M. HlznNo Llnnv W. FrNcBn GeophysicalLaboratory and Centerfor High-PressureResearch, Carnegie Institution of Washington, 5251Broad Branch Road NW, Washington,DC 20015-1305,U.S.A. ABSTRACT We have studied cubanite, CuFerS, (orthorhombic, space gtoup Pcmn, a: 6.46, b : 11.10, c : 6.22 A), using single-crystalX-ray diffraction in a diamond-anvil cell at room temperature from 0 to 3.68 GPa. Refinementswere performed aI 0, 1.76, and 3.59 GPa, and cell parameterswere measuredat 20 pressuresup to 3.68 GPa. The linear compress- ibilities of the a, b, and c crystalaxes are 0.00513(5),0.00479(5),and 0.00575(4)GPa ', respectively.Compressibility data were fitted to a Birch-Murnaghan equation of statewith parametersKo : 55.3 + 1.7 GPa with Ko' constrained to be 4. High-pressurerefinement data indicate that the principal responseof the crystal structure to compression is a re- duction in Cu-S bond lengths, whereasFe-S bond lengths remain essentiallyunchanged. Results are compared to bulk moduli and polyhedral bulk moduli of other sulfides. INrnonucrroN lography provides an excellent means for examining the effect of pressure on individual atomic configurations. The crystal structure of cubanite, CuFerSr, is ortho- Cubanite is a worthy candidate becauseof the interest in rhombic with ordered tetrahedral cation sites and S at- the effect ofpressure on intervalence transitions (see,for oms in approximately hexagonal closest packing. The example,Burns, I 98 l) and becausevery few sulfideshave structure was first determined by Buerger (1945, 1947) beenexamined with this technique.We undertook a crys- and described as slices of wurtzite structure parallel to tallographic study of cubanite at high pressurewith the (010) with widthb/2,joined such that every other slab is following goals: (l) to determine the relative axial com- inverted, resulting in edge-sharedpairs oftetrahedra. -

EXPERIMENTAL STUDY of CRYSTALLIZATION PRODUCTS of CНALCOPYRITE SOLID SOLUTION Tatyana A
86 New Data on Minerals. 2011. Vol. 46 EXPERIMENTAL STUDY OF CRYSTALLIZATION PRODUCTS OF CНALCOPYRITE SOLID SOLUTION Tatyana A. Kravchenko Institute of Mineralogy and Petrography Siberian Branch RAS, Novosibirsk, [email protected] In order to understand the conditions of formation of cubanite СuFe2S3, talnakhite Cu9Fe8S16, mooihoekite Cu9Fe9S16 and haycockite Cu4Fe5S8 in magmatic Cu-Fe ores of the Norilsk type the method of melt cooling from 1150–1100°C up to room temperature and subsequent annealing at 600 and 800°C phase associations of the cen- tral part of Cu-Fe-S system have been synthesized: 50 at.% of S, Cu/Fe = 1.22–0.25, 47 at.% S, Cu/Fe = 1.12–0.63 and 45 at.% S, Cu/Fe = 1.44–0.69. According to the received results, cubic cubanite enriched in cop- per (Cu/Fe і 0.5) crystallizes in associations with tetragonal chalcopyrite Cu1xFe1+xS2 and cubic talnakhite. The new data concerning steady phase equillibriums of mooihoekite with bornite Cu5FeS4 and cubic pc phase of the haycockite composition with cubic cubanite enriched in iron (Cu/Fe Ј 0.5) bornite and pyrrhotite Fe1xS are received. 1 figure, 1 table, 20 references. Key words: Cu-Fe-S system, chalcopyrite solid solution, crystallization of melt. Introduction system for determination of phase composi- tions and phase equillibriums in the sphere of Cubic talnakhite Cu9Fe8S16 (Bud`ko, Kula - chalcopyrite solid solution. gov, 1963; Cabri, 1967), tetragonal mooihoekite Cu9Fe9S16 (Cabri, Hall, 1972; Muraviova et al., Methodics 1972), rhombic haycockite Cu4Fe5S8(Cabri, Hall, 1972) and cubic cubanite CuFe2S3 (Cabri, 1973) Up to now the most complete experimental are attributed to the products of crystallization of research of phases from the sphere of chalcopy- chalcopyrite (Yund, Kullerud, 1966) intermedi- rite solid solution is the work by L.J. -

Troilite Fes C 2001-2005 Mineral Data Publishing, Version 1
Troilite FeS c 2001-2005 Mineral Data Publishing, version 1 Crystal Data: Hexagonal. Point Group: 6/m 2/m 2/m. Massive, granular; nodular. Physical Properties: Hardness = 3.5–4.5 VHN = 250(3) D(meas.) = 4.67–4.79 D(calc.) = 4.85 Optical Properties: Opaque. Color: Pale grayish brown, tarnishes rapidly on exposure. Streak: Dark grayish brown. Luster: Metallic. Anisotropism: Strong. R1–R2: (400) 24.3–28.4, (420) 24.9–29.6, (440) 25.8–30.9, (460) 27.1–32.6, (480) 28.4–34.0, (500) 29.9–35.5, (520) 31.3–36.7, (540) 32.7–37.9, (560) 34.2–39.0, (580) 35.5–40.0, (600) 36.9–41.0, (620) 38.1–41.7, (640) 39.2–42.4, (660) 40.2–43.1, (680) 41.1–43.5, (700) 42.0–44.0 Cell Data: Space Group: P 63/mmc. a = 5.958 c = 11.74 Z = 12 X-ray Powder Pattern: Del Norte Co., California, USA. 2.09 (100), 2.66 (60), 1.719 (50), 2.98 (40), 1.331 (40), 1.119 (40), 1.923 (30) Chemistry: (1) (2) (3) Fe 62.70 63.0 63.53 S 35.40 35.0 36.47 Total 98.10 98.0 100.00 (1) Del Norte Co., California, USA. (2) Cranbourne meteorite. (3) FeS. Occurrence: In serpentine (Del Norte Co., California, USA); with Fe–Cu–Ni sulfides in a layered ultramafic intrusive (Sally Malay deposit, Australia); and as nodules in meteorites. Association: Pyrrhotite, pentlandite, mackinawite, cubanite, valleriite, chalcopyrite, pyrite (Wannaway deposit, Australia); daubr´eelite,chromite, sphalerite, graphite, various phosphates and silicates (meteorites). -

149. Crystal Structure of Enargite (Cu3ass4)
524 [Vol. 9, 149. Crystal Structure of Enargite (Cu3AsS4). By Katsutoshi TAKANE. Institute of Mineralogy, Petrology and Economic Geology, Tohoku Imperial University, Sendai. (Rec. Nov. 11, 1933. Comm. by S. Kozu, M.I.A., Nov. 13, 1933.) Recently, the crystal structures of copper sulphides such as covellite (CuS), wolfsbergite (CuSbS2), emplectite (CuBiS2), chalcopyrite (CuFeS2) and sulvanite (Cu3VS4), have been worked out by different authors. Among these minerals, sulvanite has been grouped in the mineral family to which enargite belongs, because of the similarity in their chemical compositions. However they are different in crystallographic nature, as sulvanite belongs the cubic system of the space group T1d, determined by Pauling and Hultgren, and enargite belongs to the orthor hombic system of the space group V12h,determined by the present author. Symmetry:-According to the morphological studies already made, enargite belongs to the orthorhombic holodedral class, the axial ratio being given as a : b : c=0.8694 : 1 : 0.8308 by Groth and Mieleitner. The Laue photograph taken from (001) shows no objection to taking the crystal as possessing the symmetry of the orthorhombic holodedral class. It is noteworthy that the photograph indicates a pseudohexagonal symmetry, of which a brief discussion has already been written in Japanese. Unit cell:-From three reflection photographs taken by rotation of three mineral rods parallel to [001], [010] and [100] respectively, immersing in the beam of the CuK ray, the distances of the layer lines were measured, and the results are The axial ratio obtained from the above figures is a : b : c=1.7341.7 1.674, which are double the values of a and c given by the goniometric method. -

Metals from Ores: an Introduction
CRIMSONpublishers http://www.crimsonpublishers.com Mini Review Aspects Min Miner Sci ISSN 2578-0255 Metals from Ores: An Introduction Fathi Habashi* Department of Mining, Laval University, Canada *Corresponding author: Fathi Habashi, Department of Mining, Metallurgical and Materials Engineering, Laval University, Quebec City, Canada Submission: October 09, 2017; Published: December 11, 2017 Introduction of metallic lustre. Of these about 300 are used industrially in the chemical industry, in building materials, in fertilizers, as fuels, etc., chemical composition, constant physical properties, and a A mineral is a naturally occurring substance having a definite characteristic crystalline form. Ores are a mixture of minerals: they are processed to yield an industrial mineral or treated chemically and are known as the industrial minerals Figure 3. to yield a single or several metals. Ores that are generally processed for only a single metal are those of iron, aluminium, chromium, tin, mercury, manganese, tungsten, and some ores of copper. Gold ores may yield only gold, but silver is a common associate. Nickel ores are always associated with cobalt, while lead and zinc always occur together in ores. All other ores are complex yielding a number of metals. before being treated by chemical methods to recover the metals. Ores undergo a beneficiation process by physical methods and grinding then separation of the individual mineral by physical Figure 2: Metals and metalloids obtained from ores. Beneficiation processes involve liberation of minerals by crushing methods (gravity, magnetic, etc.) or physicochemical methods pyrometallurgical, and electrochemical methods. Metals and (flotation) Figure 1. Chemical methods involve hydrometallurgical, metalloids obtained from ores are shown in Figure 2. -

Download the Scanned
Tnn AuERTcAN M rxuRAr,ocrsr JOURNAL OT'THE MINERALOGICAL SOCIETY OF AMERICA Vol. 19 JULY, 1934 No. 7 CRYSTALLOGRAPHIC RELATIONS BETWEEN CUBAN- ITE SEGREGATION PLATES, CHALCOPYRITE MATRIX, AND SECONDARY CHALCO- PYRITE TWINS N. W. Buancnn eNn M. J. Burncrn, M assachusettsInstitute of T echnology, Cambrid. ge, M ass. ABsrnncr The orientation of cubanite plates unmixed from chalcopyrite have been said to parallel | 111 | of the chalcopyrite matrix, but the best method hitherto used to determine this does not lead to a unique solution. rn this paper, the orientations of the cubanite plates as well as certain twin lamellae, occurring in the coarse grained chalcopyrite from Corinth, Vermont, are uniquely determined by a study of the traces of these features on two polished surfaces. The general method of determining the pole of a plane making traces on two datum planes is discussed with the aid of the stereographic projection. The method is then applied to the cubanite and twin lamellae traces, resulting in a composite stereographic projection of these features. The orientation of the chalcopyrite crystal containing these is determined from the symmetry of this composite pro- jection. This, in turn, permits the poles to be easily indexed. cubanite plates are found to be parallei to {1111, as previously surmised. Two new twin lamellae are discovered, the first, parallel to {1101, called grid twins, the second parallel to { 101 }, called erhelon twins, the names being desciiptive of the habit of the lamellae. Both groups of lamellae are secondary, resulting from plastic deformation. The possible causes of this include external forces, unmixing stresses) and possible in- version stresses.