Metals from Ores: an Introduction
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Paolovite Pd2sn C 2001-2005 Mineral Data Publishing, Version 1
Paolovite Pd2Sn c 2001-2005 Mineral Data Publishing, version 1 Crystal Data: Orthorhombic. Point Group: 2/m 2/m 2/m. As irregular grains embedded in other minerals. Twinning: Polysynthetic. Physical Properties: Hardness = n.d. VHN = 329–378 (10 g load). D(meas.) = n.d. D(calc.) = 11.08 Optical Properties: Opaque. Color: Lilac-rose. Luster: Metallic. Pleochroism: Dark lilac-rose to pale rose. R1–R2: (400) — , (420) 44.4–46.5, (440) 44.6–47.2, (460) 45.0–48.2, (480) 45.5–49.1, (500) 45.9–50.3, (520) 46.7–51.6, (540) 47.7–52.8, (560) 49.0–54.1, (580) 50.9–55.4, (600) 52.9–56.7, (620) 55.3–57.7, (640) 57.7–59.2, (660) 59.8–59.5, (680) 61.5–60.3, (700) 62.7–60.6 Cell Data: Space Group: P bnm. a = 8.11(1) b = 5.662(6) c = 4.324(2) Z = 4 X-ray Powder Pattern: Oktyabr deposit, Russia. 2.28 (100), 2.16 (70), 1.955 (50), 2.36 (40), 1.397 (40), 1.315 (40), 1.120 (40) Chemistry: (1) (2) (3) Pd 64.8 64.3 64.19 Pt 2.5 Sn 35.5 35.0 35.81 Sb 0.3 Bi 0.2 Total 103.3 99.3 100.00 (1) Oktyabr deposit, Russia; by electron microprobe, corresponding to (Pd1.98Pt0.04)Σ=2.02Sn0.98. (2) Western Platinum mine, South Africa; by electron microprobe, corresponding to Pd2.02Sn0.98. (3) Pd2Sn. Occurrence: In Cu–Ni sulfide ores; in cubanite–chalcopyrite, cubanite–talnakhite, and cubanite–mooihoekite assemblages (Oktyabr deposit, Russia). -

Cu-Fe-S Phases in Lunar Rocks
American Mineralogist, Volume 58, pages 952-954, 1973 Cu-Fe-SPhases in LunarRocks LawnnNcn A. Tevronl Departmentol Geosciences,Purdue Uniuersity, Lalaye t te, I ndiana47 907 KBNNrrn L. Wnrrnus Departmentof Applied Earth Sciences,Stanlord Uniaersity, Stanford, Calilornia 943 0 5 Abstract The sulfide minerals reported to be present in lunar rocks include troilite, mackinawite, the discredited mineral "chalcopyrrhotite", sphalerite, chalcopyrite, and cubanite. Apollo 12 sam- ple l2D2l,l34 contains these last two minerals, and the first chemical analyses of the Cu-Fe-S phases are presented. These minerals occur along cracks and grain boundaries within troilite and are probably exsolution products formed at low temperatures (i.e, 100"-300'C) from a cupriferous troilite. Introduction Cu-Fe-S Minerals The opaqueminerals, although constituting only a "Chalcopyrrhotite", a discreditedmineral (Yund minor amount of a given sample,have proven useful and Kullerud, 1966; Cabri, 7967) , hasbeen reported as indicators of the genesisand cooling histories of from Apollo 12, 14, and 15 rocks (El Goresyet al, the lunar rocks (e.9., Reid, 1971; Taylor and Mc- l97la,b; 1972a,b). It wasdescribed as a yellowish, Callister, 19721,Taylor et al, I973b). The most chalcopyrite-coloredphase which appearsisotropic abundant opaque mineral is ilmenite, and many of and is alwaysfound within troilite. It is probablethat the oxide phasesin lunar rocks are unique (e.6'., this phase is either talnakhite, a mineral originally armalcolite as per Anderson et al, l97A). In addi- describedby Cabri (1967) andhaving a composition tion to native Fe metal, native Cu, and schreibersite, of CusFesSro(Cabri and Harris, 1971) near chal- the remaining opaqueminerals consistof sulfides. -

MACKINAWITE from SOUTH AFRICA W. C J. Van RDNSBU*.O, Geological Surtey Oj Soulh Africa, Preloria, Soulh A.[Ri Co, L. Lrbnunsbyc
TI.IE AMERICAN MINERALOGIST, VOL 52, JULY AUGUST, 1967 MACKINAWITE FROM SOUTH AFRICA W. C J. vAN RDNSBU*.o,Geological Surtey oJ Soulh Africa, Preloria,Soulh A.[rico, AND L. LrBnuNsByc, Deparlment of Geology,Llnit:ersity of Pretoria, Pre!oria,Soul h Africa. ABSTRAcT Mackinawite has been identified in mafic and ultramafic rocks of the Bushveld igneous complex and Insizwa and also in the carbonatite and pegmatoid of Loolekop, Phalaborrva complex in South Africa. The mineral occurs predominantly as somewhat irregular to oriented intergrowths in pentlandite in all these occurrences and less commonly as regular oriented lamellae in chalcopyrite and rarely in cubanite. The textural evidence suggests that mackinawite may represent an exsolution product of pentlandite, chalcopyrite and cubanite. INrnonucrroN The iron sulphide, mackinawite was recently named in a paper by Evans, et al, (1964). Natural occurrencesof this mineral f rom Finland were describedby- Kouvo et al (1963)and from the Muskox intrusion in Canada by Chamberlain and Delabio (1965). During the presentinvestigation mackinawite was distinguishedfrom valleriite in the carbonatite and pegmatoid of Loolekop, Phalaborwa complex, in the Bushveld igneous complex, and from Insizwa, Cape Province.The mode of occurrenceof the mackinawite in the carbonatite difiers somewhatfrom that in the mafic rocks of Insizwa and the Bush- veld complex. MrNnn.q.rocv The physicaland optical propertiesof mackinawitefrom the Bushveid complex,Insizwa and Loolekop are very similar and are in closeagree- ment with the propertiesgiven for the same mineral from the Muskox intrusion by Chamberlain and Delabio (1965). The mineral takes a fairly good polish,particularly after buffing with a chromic oxide slurry. -

Mineral Processing
Mineral Processing Foundations of theory and practice of minerallurgy 1st English edition JAN DRZYMALA, C. Eng., Ph.D., D.Sc. Member of the Polish Mineral Processing Society Wroclaw University of Technology 2007 Translation: J. Drzymala, A. Swatek Reviewer: A. Luszczkiewicz Published as supplied by the author ©Copyright by Jan Drzymala, Wroclaw 2007 Computer typesetting: Danuta Szyszka Cover design: Danuta Szyszka Cover photo: Sebastian Bożek Oficyna Wydawnicza Politechniki Wrocławskiej Wybrzeze Wyspianskiego 27 50-370 Wroclaw Any part of this publication can be used in any form by any means provided that the usage is acknowledged by the citation: Drzymala, J., Mineral Processing, Foundations of theory and practice of minerallurgy, Oficyna Wydawnicza PWr., 2007, www.ig.pwr.wroc.pl/minproc ISBN 978-83-7493-362-9 Contents Introduction ....................................................................................................................9 Part I Introduction to mineral processing .....................................................................13 1. From the Big Bang to mineral processing................................................................14 1.1. The formation of matter ...................................................................................14 1.2. Elementary particles.........................................................................................16 1.3. Molecules .........................................................................................................18 1.4. Solids................................................................................................................19 -

Understanding Fluid–Rock Interactions and Lixiviant/Oxidant Behaviour for the In-Situ Recovery of Metals from Deep Ore Bodies
School of Earth and Planetary Science Department of Applied Geology Understanding Fluid–Rock Interactions and Lixiviant/Oxidant Behaviour for the In-situ Recovery of Metals from Deep Ore Bodies Tania Marcela Hidalgo Rosero This thesis is presented for the degree of Doctor of Philosophy of Curtin University February 2020 1 Declaration __________________________________________________________________________ Declaration To the best of my knowledge and belief, I declare that this work of thesis contains no material published by any other person, except where due acknowledgements have been made. This thesis contains no material which has been accepted for the award of any other degree or diploma in any university. Tania Marcela Hidalgo Rosero Date: 28/01/2020 2 Abstract __________________________________________________________________________ Abstract In-situ recovery (ISR) processing has been recognised as a possible alternative to open- pit mining, especially for low-grade resources. In ISR, the fluid–rock interaction between the target ore and the lixiviant results in valuable- (and gangue-) metal dissolution. This interaction is achieved by the injection and recovery of fluid by means of strategically positioned wells. Although the application of ISR has become more common (ISR remains the preferential processing technique for uranium and has been applied in pilot programs for treating oxide zones in copper deposits), its application to hard-rock refractory and low-grade copper-sulfide deposits is still under development. This research is focused on the possible application of ISR to primary copper sulfides usually found as deep ores. Lixiviant/oxidant selection is an important aspect to consider during planning and operation in the ISR of copper-sulfide ores. -
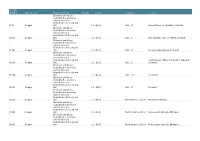
BRSUG Number Mineral Name Hey Index Group Hey No
BRSUG Number Mineral name Hey Index Group Hey No. Chem. Country Locality Elements and Alloys (including the arsenides, antimonides and bismuthides of Cu, Ag and B-37 Copper Au) 1.1 4[Cu] U.K., 17 Basset Mines, nr. Redruth, Cornwall Elements and Alloys (including the arsenides, antimonides and bismuthides of Cu, Ag and B-151 Copper Au) 1.1 4[Cu] U.K., 17 Phoenix mine, Cheese Wring, Cornwall Elements and Alloys (including the arsenides, antimonides and bismuthides of Cu, Ag and B-280 Copper Au) 1.1 4[Cu] U.K., 17 County Bridge Quarry, Cornwall Elements and Alloys (including the arsenides, antimonides and bismuthides of Cu, Ag and South Caradon Mine, 4 miles N of Liskeard, B-319 Copper Au) 1.1 4[Cu] U.K., 17 Cornwall Elements and Alloys (including the arsenides, antimonides and bismuthides of Cu, Ag and B-394 Copper Au) 1.1 4[Cu] U.K., 17 ? Cornwall? Elements and Alloys (including the arsenides, antimonides and bismuthides of Cu, Ag and B-395 Copper Au) 1.1 4[Cu] U.K., 17 Cornwall Elements and Alloys (including the arsenides, antimonides and bismuthides of Cu, Ag and B-539 Copper Au) 1.1 4[Cu] North America, U.S.A Houghton, Michigan Elements and Alloys (including the arsenides, antimonides and bismuthides of Cu, Ag and B-540 Copper Au) 1.1 4[Cu] North America, U.S.A Keweenaw Peninsula, Michigan, Elements and Alloys (including the arsenides, antimonides and bismuthides of Cu, Ag and B-541 Copper Au) 1.1 4[Cu] North America, U.S.A Keweenaw Peninsula, Michigan, Elements and Alloys (including the arsenides, antimonides and bismuthides of Cu, -

Geochemistry, Mineralogy and Microbiology of Cobalt in Mining-Affected Environments
minerals Article Geochemistry, Mineralogy and Microbiology of Cobalt in Mining-Affected Environments Gabriel Ziwa 1,2,*, Rich Crane 1,2 and Karen A. Hudson-Edwards 1,2 1 Environment and Sustainability Institute, University of Exeter, Penryn TR10 9FE, UK; [email protected] (R.C.); [email protected] (K.A.H.-E.) 2 Camborne School of Mines, University of Exeter, Penryn TR10 9FE, UK * Correspondence: [email protected] Abstract: Cobalt is recognised by the European Commission as a “Critical Raw Material” due to its irreplaceable functionality in many types of modern technology, combined with its current high-risk status associated with its supply. Despite such importance, there remain major knowledge gaps with regard to the geochemistry, mineralogy, and microbiology of cobalt-bearing environments, particu- larly those associated with ore deposits and subsequent mining operations. In such environments, high concentrations of Co (up to 34,400 mg/L in mine water, 14,165 mg/kg in tailings, 21,134 mg/kg in soils, and 18,434 mg/kg in stream sediments) have been documented. Co is contained in ore and mine waste in a wide variety of primary (e.g., cobaltite, carrolite, and erythrite) and secondary (e.g., erythrite, heterogenite) minerals. When exposed to low pH conditions, a number of such minerals are 2+ known to undergo dissolution, typically forming Co (aq). At circumneutral pH, such aqueous Co can then become immobilised by co-precipitation and/or sorption onto Fe and Mn(oxyhydr)oxides. This paper brings together contemporary knowledge on such Co cycling across different mining environments. -

Handbook of Iron Meteorites, Volume 2 (Bitburg
326 Bishop Canyon - Bitburg fibrous and brecciated, and 1-100 p wide veins through it ' l ' j are filled with a glass in which silicate fragments and a little ) ·. / troilite are embedded.lt is surprising to note how the shock . e·· f . has been able to create such heavy local transformation; 10 mm away nothing unusual is observed. The local damage resembles in several respects the structural changes associated with spot welding. Examination under the electron microprobe suggests that the unknown silicate is ~' almost pure Si02 , possibly tridymite. Bishop Canyon is chemically and structurally a typical ~ phosphorus-poor, fine octahedrite of groupiVA, . l .. resembling in particular Gibe on. It is, however, unusual in .0 its silicate inclusions, the shock damage and low hardness. ,, .• ~: :t • ·. • cr ··. @ Specimen in the U.S. National Museum in Washington: .. ~ - ,·r -. f '-' f 226 g slice (no. 770, 9.5 x 4.5 x 0.9 em) II . 0 t' / •. ' ~~ ~~ {) r ...~ · -· · ...· ~· -/~... Figure 352. Bitburg. Detail of Figure 350. The dendritic metal was Bitburg, Rhineland, Germany at high temperature austenite but transformed upon cooling to unequilibrated a • Etched. Scale bar 50 f.l. 49°58'N, 6°32'E 2 A mass said to have weighed 1.5 tons was smelted in a furnace before 1805. It was recognized as a meteorite (Chladni 1819: 353), but virtually nothing was saved of the undamaged material (Hey 1966: 57). The smelted material, e.g., no. 1881, 1534 of 546 gin .. \ . • I : '/ 'I \ ' . ~\.. // .·· Figure 350. Bit burg (Copenhagen no. 1886, 493). A rectangular bar cut from melted material of the Bitburg meteorite. -

Plagioclase-Spinel-Graphite Xenoliths in Metallic Iron
THE AMERICAN MINERALOGIST, VOL. 51, MAY_JUNE, 1966 PLAGIOCLASE-SPINEL-GRAPHITEXENOLITHS IN METALLIC IRON-BEARING BASALTS, DISKO ISLAND, GREENLAND Wrr,rrau G. MBr.soNeNo Gnoncp Swrrzrn Departmentof Mineral Sciences, U. S. l{ational Museum,Washington, D. C. Ansrnac:t Tertiary basalt flows from west Greenland commonly contain metallic iron and graph- ite. They are thus some of the most reduced of terrestrial basalts, and are of interest in con- nection with basalt studies in general, and in connection with the origin of certain meteor- ites (Lovering, 1964). Xenoliths composed mainly of plagioclase (Anzo-zu), graphite, rose-colored spinel and rarely corundum are included in these basalts on Disko Island. The xenoliths are remark- ably similar to reconstituted shale xenoliths in basaltic rocks from other localities except for the presence oI graphite and in the composition of the spinel. The spinel has n:1.747 +0.001, o:8'119+0.002 and D:3.81 +0.01, and electron microprobe analysis gave Mg : 13 and Fe:8.9/6. These data indicate a spinel with composition about spzaHezz.This un- usually low Fe content compared to spinels from other aluminous xenoliths in basaltic rocks is evidently related to the low oxygen fugacities ln basaltic magmas in contact with graphite, particularly in an environment such as Disko, where active reduction of ferrous iron to iron evidently occurred. The close similarities in the mineralogy of the xenoliths from specimen to specimen indi- cate complete equilibration of magma and xenoliths. This equilibration involved mainly loss of K and Si, and gain of Ca, Mg, Al and Na by the xeno.liths. -

Manganese Deposits Near Bromide, Oklahoma
MANGANESE DEPOSITS NEAR BROMIDE, OKLAHOMA. By D. F. HEWETT. FIELD WORK. During 1917, when there was a prospective shortage of man ganese ore, the attention of the United States Geological Survey was called to manganese deposits near Bromide, Johnston County, Okla. In October of that year it was possible for the writer, in company with George E. Burton, then assistant director of the Oklahoma Geological Survey, to devote three days to the exam ination of the deposits. Although the deposits are small and can not yield large quantities of high-grade ore, their relations and the minerals they contain are so uncommon that a record of their features is warranted. SITUATION AND ACCESSIBILITY. Five manganese deposits or groups of deposits are known near Bromide. The most extensive and probably the largest deposit is near the settlement of Springbrook (formerly Viola), in the SW. I sec. 13, T. 2 S., R,. 7 E., about 4 miles southwest of Bromide. The other deposits lie along the valley of Moseley Creek, from 1 to 5 miles northeast of Bromide, in the SW. £ sec. 28, the NE. $ sec. 20, the SE. i sec. 17, and the NE. i sec. 17, T. 1 S., R. 8 E. Bromide is 6 miles northwest of Wapanucka, with which it is connected by a spur of the Missouri, Oklahoma & Gulf Railway. (See fig. 48.) Bromide lies at the northern edge of the broad valley of Delaware Creek, near the eastern limit of the Arbuckle Mountains. In this region the mountains consist of a poorly defined dissected plateau that attains an altitude of about 1,000 feet above sea level several miles northwest of Bromide. -

Download the Scanned
American Mineralogist, Volume 77, pages 670475, 1992 NEW MINERAL NAMES* JonN L. J,Annson CANMET, 555 Booth Street,Ottawa, Ontario KIA OGl' Canada Abswurmbachite* rutile, hollandite, and manganoan cuprian clinochlore. The new name is for Irmgard Abs-Wurmbach, in recog- T. Reinecke,E. Tillmanns, H.-J. Bernhardt (1991)Abs- her contribution to the crystal chemistry, sta- wurmbachite, Cu'?*Mnl*[O8/SiOo],a new mineral of nition of physical properties ofbraunite. Type the braunite group: Natural occurrence,synthesis, and bility relations, and crystal structure.Neues Jahrb. Mineral. Abh., 163,ll7- material is in the Smithsonian Institution, Washington, r43. DC, and in the Institut fiir Mineralogie, Ruhr-Universitlit Bochum, Germany. J.L.J. The new mineral and cuprian braunit€ occur in brown- ish red piemontite-sursassitequartzites at Mount Ochi, near Karystos, Evvia, Greece, and in similar quartzites on the Vasilikon mountains near Apikia, Andros Island, Barstowite* Greece.An electron microprobe analysis (Andros mate- C.J. Stanley,G.C. Jones,A.D. Hart (1991) Barstowite, gave SiO, 9.8, TiO, rial; one of six for both localities) 3PbClr'PbCOr'HrO, a new mineral from BoundsClifl 0.61,Al,O3 0.60, Fe'O, 3.0,MnrO. 71.3,MgO 0.04,CuO St. Endellion,Cornwall. Mineral. Mag., 55, l2l-125. 12.5, sum 97.85 wto/o,corresponding to (CuStrMn3tu- Electron microprobe and CHN analysis gavePb75.47, Mgoo,)", oo(Mn3jrFe|jrAlo orTif.[nCuStr)", nrSi' o, for eight (calc.)6.03, sum 101.46wto/o, cations,ideally CuMnuSiO'r, the Cu analogueof braunite. Cl 18.67,C l.Iz,H 0.18,O to Pb.orClrrrCr.or- The range of Cu2* substitution for Mn2' is 0-42 molo/oin which for 17 atoms corresponds The min- cuprian braunite and 52-93 molo/oin abswurmbachite. -

The Tawallah Valley Meteorite. General Description. the Microstructure.Records of the Australian Museum 21(1): 1–8, Plates I–Ii
AUSTRALIAN MUSEUM SCIENTIFIC PUBLICATIONS Hodge-Smith, T., and A. B. Edwards, 1941. The Tawallah Valley meteorite. General description. The Microstructure.Records of the Australian Museum 21(1): 1–8, plates i–ii. [4 July 1941]. doi:10.3853/j.0067-1975.21.1941.518 ISSN 0067-1975 Published by the Australian Museum, Sydney nature culture discover Australian Museum science is freely accessible online at http://publications.australianmuseum.net.au 6 College Street, Sydney NSW 2010, Australia THE TAW ALLAH VALLEY METEORITE. General Description. By T. HODGE-SMI'l'H, The Australian Museum. The Microstructure. By A. B. EDWARDS, Ph.D., D.I.C.,* Research Officer, Mineragraphy Branch, Council for Scientific and Industrial Research. (Plates i-ii and Figures 1-2.) General Description. Little information is available about the finding of this meteorite. Mr. Heathcock, Constable-in-Charge of the Borroloola Police Station, Northern Territory, informed me in April, 1939, that it had been in the Police Station for eighteen months or more. It was found by Mr. Condon, presumably some time in 1937. The weight of the iron as received was 75·75 kg. (167 lb.). A small piece had been cut off, but its weight probably did not exceed 200 grammes. The main mass weighing 39·35 kg. (86i lb.) is in the collection of the Geological Survey, Department of the Interior, Canberra. A portion weighing 30·16 kg. (66~ lb.) and five pieces together weighing 1·67 kg. are in the collection of the Australian Museum, and a slice weighing 453 grammes is in the Museum of the Geology Department, the University of Melbourne.