Skin Cancer & Summer Sun Tips
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

GAO-18-61, SUNSCREEN: FDA Reviewed Applications For
United States Government Accountability Office Report to Congressional Committees November 2017 SUNSCREEN FDA Reviewed Applications for Additional Active Ingredients and Determined More Data Needed GAO-18-61 November 2017 SUNSCREEN FDA Reviewed Applications for Additional Active Ingredients and Determined More Data Needed Highlights of GAO-18-61, a report to congressional committees Why GAO Did This Study What GAO Found Using sunscreen as directed with other The Food and Drug Administration (FDA), within the Department of Health and sun protective measures may help Human Services, implemented requirements for reviewing applications for reduce the risk of skin cancer—the sunscreen active ingredients within time frames set by the Sunscreen Innovation most common form of cancer in the Act, which was enacted in November 2014. For example, the agency issued a United States. In the United States, guidance document on safety and effectiveness testing in November 2016. sunscreen is considered an over-the- counter drug, which is a drug available As of August 2017, all applications for sunscreen active ingredients remain to consumers without a prescription. pending after the agency determined more safety and effectiveness data are Some sunscreen active ingredients not needed. By February 2015, FDA completed its initial review of the safety and currently marketed in the United States effectiveness data for each of the eight pending applications, as required by the have been available in products in act. FDA concluded that additional data are needed to determine that the other countries for more than a ingredients are generally recognized as safe and effective (GRASE), which is decade. Companies that manufacture needed so that products using the ingredients can subsequently be marketed in some of these ingredients have sought the United States without FDA’s premarket approval. -
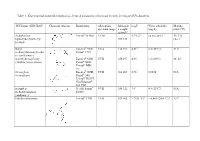
Table 1 - Experimental and Predicted Physical-Chemical Parameters of the Most Recently Investigated UV-Absorbers
Table 1 - Experimental and predicted physical-chemical parameters of the most recently investigated UV-absorbers. INCI name (INN/XAN) Chemical structure Brand name Absorption Molecula LogP Water solubility Melting spectrum range r weight (mg/L) point (°C) (g/mol)4 diethylamino Uvinul® A Plus UVA1 5.7-6.21 <0.01 (20°C) 1 54; 314 hydroxybenzoyl hexyl 397.515 (dec.) 1 benzoate Butyl Eusolex® 9020, UVA 310.393 4.514 2.2 (25°C)4 83.54 methoxydibenzoylmetha Parsol® 1789 ne (avobenzone) 4-methylbenzylidene Eusolex® 6300 UVB 258.397 4.95 1.3 (20°C) 66–68 camphor (enzacamene) Parsol® 5000 Uvinul® MBC 95 Octocrylene Eusolex® OCR, UVB 361.485 6.783 0.00383 N/A (octocrilene) Parsol® 340, Uvinul® N539T, NeoHeliopan® 303 USP isoamyl p- Neo Heliopan® UVB 248.322 3.61 4.9 (25°C)1 N/A methoxycinnamate E1000 (amiloxate) Ethylhexyl triazone Uvinul® T150 UVB 823.092 > 7(20 °C) 6 < 0.001 (20.0 °C) 6 1296 Ethylhexyl Parsol® MCX, UVB 290.403 6.14 0.041 (24 °C and N/A methoxycinnamate Heliopan® New pH 7.1) 4 (octinoxate) Ethylhexyl dimethyl Escalol™ 507 UVB 277.4084 5.774 0.54 (25 °C) 4 N/A PABA (padimate-O) Arlatone 507 Eusolex 6007 benzophenone-3 Eusolex® 4360 UVA2+ UVB 228.247 3.72 3.7 (20°C) 2 62-652 (oxybenzone) bis-ethylhexyloxyphenol Tinosorb® S UVA1+UVB 627.826 12.61 <10-4 80.401 methoxyphenol triazine (bemotrizinol) Phenylbenzimidazole Eusolex® 232 UVA2+ UVB 274.2945 -1.1 (pH 5) > 30% (As sodium N/A sulfonic acid Parsol® HS -2.1 (pH 8)5 or (ensulizole) Neo Heliopan® triethanolammoniu Hydro m salt at 20°C) 5 1 (3) 2 (34) 3 (44) 4 Pubchem 5 SCCP/1056/06 Opinion on phenylbenzimidazole sulfonic acid and its salts 6 BASF safety data sheet Table 2 – In vitro studies for the assessment of skin permeation/penetration of sunscreens. -

Photophysics and Skin Penetration of Active Agents in a Commercial Sunscreen and Insect Repellent
PHOTOPHYSICS AND SKIN PENETRATION OF ACTIVE AGENTS IN A COMMERCIAL SUNSCREEN AND INSECT REPELLENT by DONALD PRETTYPAUL A Dissertation submitted to the Graduate School-Newark Rutgers, The State University of New Jersey In partial fulfillment of the requirements for the degree of Doctor of Philosophy Graduate Program in Chemistry written under the direction of Professor Richard Mendelsohn Professor Piotr Piotrowiak and approved by Newark, New Jersey October 2018 ©2018 Donald Prettypaul ALL RIGHTS RESERVED ABSTRACT OF DISSERTATION PHOTOPHYSICS AND SKIN PENETRATION OF ACTIVE AGENTS IN A COMMERCIAL SUNSCREEN AND INSECT REPELLENT By DONALD PRETTYPAUL Dissertation co-Directors: Professor Richard Mendelsohn Professor Piotr Piotrowiak This dissertation is focused on active agents in commercial sunscreen and insect repellent products. It consists of two parts, the first focusing on the photophysics of a sunscreen active agent and the second on the permeation and spatial distribution of the sunscreen active and an insect repellent active when these agents are applied to ex-vivo human skin. In the photochemistry study, ultrafast spectroscopy was used to study the excited state dynamics of the sunscreen molecule, Bemotrizinol. The work focused on the dissipation rates of the electronic excitation energy in different solvents. To complement the results from time-resolved femtosecond spectroscopy, Hartree- Fock UH/UHF 6-31G* calculations were used to characterize the ground and excited states potential energy surfaces. The results indicate that the excited state deactivation pathway follows a proton coupled electron transfer process which ii proceeds via a concerted mechanism. The dependencies on solvent polarity, viscosity, and H/D isotope effects, were investigated. Sunscreen products have been developed to protect skin from ultraviolet (UV) radiation; to achieve adequate protection, the sunscreen must be evenly applied and remain on the surface of the skin. -

FDA Proposes Sunscreen Regulation Changes February 2019
FDA Proposes Sunscreen Regulation Changes February 2019 The U.S. Food and Drug Administration (FDA) regulates sunscreens to ensure they meet safety and eectiveness standards. To improve the quality, safety, and eectiveness of sunscreens, FDA issued a proposed rule that describes updated proposed requirements for sunscreens. Given the recognized public health benets of sunscreen use, Americans should continue to use broad spectrum sunscreen with SPF 15 or higher with other sun protective measures as this important rulemaking eort moves forward. Highlights of FDA’s Proposals Sunscreen active ingredient safety and eectiveness Two ingredients (zinc oxide and titanium dioxide) are proposed to be safe and eective for sunscreen use and two (aminobenzoic acid (PABA) and trolamine salicylate) are 1 proposed as not safe and eective for sunscreen use. FDA proposes that it needs more safety information for the remaining 12 sunscreen ingredients (cinoxate, dioxybenzone, ensulizole, homosalate, meradimate, octinoxate, octisalate, octocrylene, padimate O, sulisobenzone, oxybenzone, avobenzone). New proposed sun protection factor Sunscreen dosage forms (SPF) and broad spectrum Sunscreen sprays, oils, lotions, creams, gels, butters, pastes, ointments, and sticks are requirements 2 proposed as safe and eective. FDA 3 • Raise the maximum proposed labeled SPF proposes that it needs more data for from SPF 50+ to SPF 60+ sunscreen powders. • Require any sunscreen SPF 15 or higher to be broad spectrum • Require for all broad spectrum products SPF 15 and above, as SPF increases, broad spectrum protection increases New proposed label requirements • Include alphabetical listing of active ingredients on the front panel • Require sunscreens with SPF below 15 to include “See Skin Cancer/Skin Aging alert” on the front panel 4 • Require font and placement changes to ensure SPF, broad spectrum, and water resistance statements stand out Sunscreen-insect repellent combination 5 products proposed not safe and eective www.fda.gov. -

Chemical UVR Absorbers
Chemical UVR Absorbers The names given in bold and used Diisopropyl methyl cinnamate Glyceryl ethyihexanoate dimethoxy- throughout this handbook are those of Empirical formula: cinnamate the International Nomenclature of C 6H22O2 Chemical names. Cosmetic Ingredients. Glyceryl octanoate dimethoxycinnamate; Chemical names: 2-propenoic acid, 3-(4-methoxyphenyl)-, 2-Propenoic acid, 3-12,4bis(1 diester with 1 ,3-dihydroxy-2-(2-ethyl-1 - methylethyphenyl-methyl ester; 2,5- oxohexyl)oxypropane diisopropyl methyl cinnamate _ lsoamyl-para-methoxycinnamate Ethyihexyl methoxycinnamate Empirical formula: Empirical formula: C151-12003 C 8H26O3 Chemical names: Cinnamates Chemical names: Amyl4-methoxycinnamate; isopentyl-4- 2-Ethylhexyl-4-methoxycin nam ate; methoxycinnamate; isopenlyl-para- Cinoxate 2-ethyl-hexyl-para-methoxycinnamate; methoxy-cinnamate; 3-(4-methoxyphenyl)- Empirical formula: para-methoxycinnamic acid, 2-ethylhexyl 2-propenoic acid, isopentyl ester Ci4HieO4 ester; 3-(4-methoxyphenyl)-2-propenoic acid, 2-ethylhexyl ester; octinoxate; octyl Trade names: Chemical names: methoxycinnamate; 2-propenoic acid, 3- Neo Heliopan type E 1000; Solarum AMC 2- Ethoxyothyl-para-methoxyci n nam ate; (4-methoxyphenyl)-2-ethylhexyl ester 2-propenoic acid, 3-(4-methoxyphery- para-A minobenzoic acids (PA BAs) 2-ethoxyethyl ester; 2-ethoxyethyl-4- Trade names: methoxycinnamate AEC Octyl Methoxycinnamate; Escalol Amyl dimethyl FABA 557; Eusolex 2292; Heliosol 3; Empirical formula: Trade names: Jeescreen OMC; Katoscreen OMC; Nec C14H21 NO2 Giv Tan F; Phiasol -

WO 2013/036901 A2 14 March 2013 (14.03.2013) P O P C T
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (10) International Publication Number (43) International Publication Date WO 2013/036901 A2 14 March 2013 (14.03.2013) P O P C T (51) International Patent Classification: (81) Designated States (unless otherwise indicated, for every A61K 8/30 (2006.01) kind of national protection available): AE, AG, AL, AM, AO, AT, AU, AZ, BA, BB, BG, BH, BN, BR, BW, BY, (21) International Application Number: BZ, CA, CH, CL, CN, CO, CR, CU, CZ, DE, DK, DM, PCT/US2012/054376 DO, DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, (22) International Filing Date: HN, HR, HU, ID, IL, IN, IS, JP, KE, KG, KM, KN, KP, 10 September 2012 (10.09.2012) KR, KZ, LA, LC, LK, LR, LS, LT, LU, LY, MA, MD, ME, MG, MK, MN, MW, MX, MY, MZ, NA, NG, NI, (25) Filing Language: English NO, NZ, OM, PA, PE, PG, PH, PL, PT, QA, RO, RS, RU, (26) Publication Language: English RW, SC, SD, SE, SG, SK, SL, SM, ST, SV, SY, TH, TJ, TM, TN, TR, TT, TZ, UA, UG, US, UZ, VC, VN, ZA, (30) Priority Data: ZM, ZW. 61/532,701 9 September 201 1 (09.09.201 1) US (84) Designated States (unless otherwise indicated, for every (71) Applicant (for all designated States except US): UNIVER¬ kind of regional protection available): ARIPO (BW, GH, SITY OF FLORIDA RESEARCH FOUNDATION, GM, KE, LR, LS, MW, MZ, NA, RW, SD, SL, SZ, TZ, INC. -

OSEQUE ZSOLE DUAL SUN BLOCK- Octinoxate Avobenzone Bisoctrizole Spray SONGHAK CO., LTD
OSEQUE ZSOLE DUAL SUN BLOCK- octinoxate avobenzone bisoctrizole spray SONGHAK CO., LTD. ---------- Active ingredients: Octyl Methoxycinnamate 7.5%, Ethylhexyl Salicylate 5%, Avobenzone 2%, Bisoctrizole 0.5% Purpose: Sunscreen Inactive Ingredients: Water, Alcohol Denat, Butylene Glycol, Glycerin, Bis-Ethylhexyloxyphenol Methoxyphenyl Triazine, Niacinamide, Isononyl Isononanoate, Glacier Water, Butyloctyl Salicylate, Xanthan Gum, Decyl Glucoside, Disodium EDTA, Methylparaben, Propylparaben, Fragrance Do not use: on wounds, rashes, dermatitis or damaged skin Keep out of reach of children. In case of accidental ingestion, seek professional assistance or contact a Poison Control Center immediately Stop use: Please stop using this product and contact a dermatologist 1. If allergic reaction or irritation occurs 2. If direct sunlight affects the area as above When using this product: Do not use other than directed Warnings: For external use only. Not to be swallowed. Avoid contact with eyes. Discontinue use if signs of irritation or rash appear Uses: Spray or apply liberally over the face and body 15 minutes prior to sun exposure. Always ensure total coverage of all sun exposed areas. Re-apply every 1-2 hours and always after swimming. For spray type, shake it well before use and spray it evenly at 20cm-30cm apart from face or wherever needed. For spray type - shake it well before use For lotion type - turn a cap to use Storage: Keep in a dry or room temperature area OSEQUE ZSOLE DUAL SUN BLOCK octinoxate avobenzone bisoctrizole spray Product -

Annex VI, Last Update: 02/08/2021
File creation date: 03/10/2021 Annex VI, Last update: 22/09/2021 LIST OF UV FILTERS ALLOWED IN COSMETIC PRODUCTS Substance identification Conditions Wording of Reference Maximum conditions of Product Type, concentration Update date number Chemical name / INN / XAN Name of Common Ingredients Glossary CAS Number EC Number Other use and body parts in ready for use warnings preparation 2 N,N,N-Trimethyl-4-(2-oxoborn-3-ylidenemethyl CAMPHOR BENZALKONIUM 52793-97-2 258-190-8 6% 15/10/2010 ) anilinium methyl sulphate METHOSULFATE 3 Benzoic acid, 2-hydroxy-, HOMOSALATE 118-56-9 204-260-8 10% 02/08/2021 3,3,5-trimethylcyclohexyl ester / Homosalate 4 2-Hydroxy-4-methoxybenzophenone / BENZOPHENONE-3 131-57-7 205-031-5 6% Reg (EU) Not more than Contains 02/08/2021 Oxybenzone 2017/238 of 10 0,5 % to protect Benzophenone-3 February 2017- product (1) date of formulation application from September 2017 6 2-Phenylbenzimidazole-5-sulphonic acid and its PHENYLBENZIMIDAZOLE SULFONIC 27503-81-7 248-502-0 8%(as acid) 08/03/2011 potassium, sodium and triethanolamine salts / ACID Ensulizole 7 3,3'-(1,4-Phenylenedimethylene) bis TEREPHTHALYLIDENE DICAMPHOR 92761-26-7 / 410-960-6 / - 10%(as acid) 26/10/2010 (7,7-dimethyl-2-oxobicyclo-[2.2.1] SULFONIC ACID 90457-82-2 hept-1-ylmethanesulfonic acid) and its salts / Ecamsule 8 1-(4-tert-Butylphenyl)-3-(4-methoxyphenyl) BUTYL 70356-09-1 274-581-6 5% 15/10/2010 propane-1,3-dione / Avobenzone METHOXYDIBENZOYLMETHANE 9 alpha-(2-Oxoborn-3-ylidene)toluene-4-sulphoni BENZYLIDENE CAMPHOR SULFONIC 56039-58-8 - 6%(as acid) -

Cutaneous Permeation and Penetration of Sunscreens: Formulation Strategies and in Vitro Methods
cosmetics Review Cutaneous Permeation and Penetration of Sunscreens: Formulation Strategies and In Vitro Methods Silvia Tampucci * ID , Susi Burgalassi ID , Patrizia Chetoni ID and Daniela Monti ID Department of Pharmacy, University of Pisa, 56126 Pisa, Italy; [email protected] (S.B.); [email protected] (P.C.); [email protected] (D.M.) * Correspondence: [email protected] Received: 1 November 2017; Accepted: 7 December 2017; Published: 25 December 2017 Abstract: Sunscreens are the most common products used for skin protection against the harmful effects of ultraviolet radiation. However, as frequent application is recommended, the use of large amount of sunscreens could reflect in possible systemic absorption and since these preparations are often applied on large skin areas, even low penetration rates can cause a significant amount of sunscreen to enter the body. An ideal sunscreen should have a high substantivity and should neither penetrate the viable epidermis, the dermis and the systemic circulation, nor in hair follicle. The research of methods to assess the degree of penetration of solar filters into the skin is nowadays even more important than in the past, due to the widespread use of nanomaterials and the new discoveries in cosmetic formulation technology. In the present paper, different in vitro studies, published in the last five years, have been reviewed, in order to focus the attention on the different methodological approaches employed to effectively assess the skin permeation and retention of sunscreens. Keywords: sunscreens; formulation; in vitro methods; cutaneous permeation; skin penetration 1. Introduction The detrimental effects of human exposure to ultraviolet (UV) radiation have been widely investigated and can be immediate, as in the case of sunburns, or long-term, causing, in most cases, the formation of oxidizing species responsible of photo-aging, immunosuppression and chronic effects such as photo carcinogenicity [1,2]. -

Sun Protection
[ Oncology Watch] Sun Protection: What We and Our Patients Need to Know To preserve their health and the appearance of their skin, patients need straight- forward, practical advice on susncreen selection and protection strategies. By Jonathan Wolfe, MD rom preventing skin cancer to pre- measures protection against cutaneous burning. Even a FDA public education serving a youthful appearance, burning—the effects of UVB—and does piece states, “A higher number means it Fsunscreens play an important role not account for UVA radiation at all.5 As protects longer.”7 This is not a strictly in dermatologic care. However, some a result, a product with a high SPF could accurate interpretation of the SPF value confusion persists among patients and actually confer little or no protection and ignores the fact that unique proper- even some practitioners when it comes to against UVA. ties of the main sunscreen ingredients SPF, available chemical and physical sun- The somewhat esoteric description of (chemical or physical) and the formula- screens, and the best advice for sun SPF calculation is available online at the tion (waterproof, water resistant) influ- avoidance. In recognition of the AAD’s FDA website.6 Ultimately, the SPF num- ence duration of protection. Skin Cancer Detection and Prevention ber represents the ratio of the MED for Patients also often falsely assume that Month, I’ll review important patient protected skin (MED(PS)) to the MED the increase in SPF value is proportion- education points. for unprotected skin (MED(US)). Tests ate to the increase in UVB protection. It are conducted only in patients with skin is not. -

Sun Protection
DRUG NEWS Recommending the Best Sun Protection Clinical Pearls: o Recommend a “broad-spectrum” sunscreen – one that covers UVB, UVA1, and UVA2. o Recommend SPF 30-50 o Advise on non-pharmacological sun protection methods o Emphasize proper sunscreen application technique o Emphasize skin protection when taking drugs known to cause photosensitivity. Familiarize yourself with known implicated drugs by referring to appendix 2. Background 1-4 The sun emits 3 types of ultraviolet (UV) radiation: UVC (100-290 nm), UVB (290-320 nm), and UVA (320 -400 nm). UVA rays can be further divided into the shorter UVA2 rays and the longer UVA1 rays. UVC rays, the shortest rays, are completely absorbed by the ozone layer, whereas UVB rays penetrate the epidermis and UVA rays, the longest rays, penetrate into the dermis. The main consequence of UVB irradiation is sunburn, but can also include immunosuppression and skin cancer. Consequences of UVA radiation include: phototoxicity (i.e. involvement in drug-induced sun sensitivity reactions), photo-aging, immunosuppression, and skin cancer. What is SPF? 2,5,6,7 It is easy to be misled by Sun Protection Factors (SPF). SPF is assessed through a standardized test by finding the ratio of the minimal dose of solar radiation that produces perceptible erythema (i.e., minimal erythema dose) on sunscreen-protected skin compared with unprotected skin. Sunburn is caused primarily by UVB rays (and shorter UVA2 rays), and thus SPF indicates mostly UVB protection. However, UVA protection is equally important since it is responsible for photo-aging and cancer. Therefore, it is important to look for the phrase “broad spectrum” when choosing a sunscreen as broad spectrum indicates both UVB and UVA protection. -

Food and Drug Administration, HHS § 352.20
Food and Drug Administration, HHS § 352.20 (c) Cinoxate up to 3 percent. than 2 to the finished product. The fin- (d) [Reserved] ished product must have a minimum (e) Dioxybenzone up to 3 percent. SPF of not less than the number of (f) Homosalate up to 15 percent. sunscreen active ingredients used in (g) [Reserved] the combination multiplied by 2. (h) Menthyl anthranilate up to 5 per- (2) Two or more sunscreen active in- cent. gredients identified in § 352.10(b), (c), (i) Octocrylene up to 10 percent. (e), (f), (i) through (l), (o), and (q) may (j) Octyl methoxycinnamate up to 7.5 be combined with each other in a single percent. product when used in the concentra- (k) Octyl salicylate up to 5 percent. tions established for each ingredient in (l) Oxybenzone up to 6 percent. § 352.10. The concentration of each ac- (m) Padimate O up to 8 percent. tive ingredient must be sufficient to (n) Phenylbenzimidazole sulfonic contribute a minimum SPF of not less acid up to 4 percent. than 2 to the finished product. The fin- (o) Sulisobenzone up to 10 percent. ished product must have a minimum (p) Titanium dioxide up to 25 percent. SPF of not less than the number of (q) Trolamine salicylate up to 12 per- sunscreen active ingredients used in cent. the combination multiplied by 2. (r) Zinc oxide up to 25 percent. (b) Combinations of sunscreen and skin [64 FR 27687, May 21, 1999] protectant active ingredients. Any single sunscreen active ingredient or any per- EFFECTIVE DATE NOTE: At 67 FR 41823, June mitted combination of sunscreen ac- 20, 2002, § 352.10 was amended by revising tive ingredients when used in the con- paragraphs (f) through (n), effective Sept.