129540588.Pdf
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
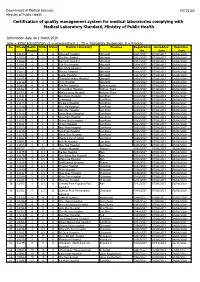
Certification of Quality Management System for Medical Laboratories Complying with Medical Laboratory Standard, Ministry of Public Health
Department of Medical Sciences F0715102 Ministry of Public Health Certification of quality management system for medical laboratories complying with Medical Laboratory Standard, Ministry of Public Health Information date on 1 March 2019 new = initial accreditation, r1 = reassessment 1 , TS = Temporary Suspension, P = Process No. HCode Health RMSc Status Medical Laboratory Province Registration Accredited Expiration Region No. Date Date 1 10673 2 2 r1 Uttaradit Hospital Uttaradit 0001/2557 07/08/2017 06/08/2020 2 11159 2 2 r1 Tha Pla Hospital Uttaradit 0002/2557 07/08/2017 06/08/2020 3 11160 2 2 r1 Nam Pat Hospital Uttaradit 0003/2557 07/08/2017 06/08/2020 4 11161 2 2 r1 Fak Tha Hospital Uttaradit 0004/2557 07/08/2017 06/08/2020 5 11162 2 2 r1 Ban Khok Hospital Uttaradit 0005/2557 07/08/2017 06/08/2020 6 11163 2 2 r1 Phichai Hospital Uttaradit 0006/2557 07/08/2017 06/08/2020 7 11164 2 2 r1 Laplae Hospital Uttaradit 0007/2557 07/08/2017 06/08/2020 8 11165 2 2 r1 ThongSaenKhan Hospital Uttaradit 0008/2557 07/08/2017 06/08/2020 9 11158 2 2 r1 Tron Hospital Uttaradit 0009/2557 07/08/2017 06/08/2020 10 10863 4 4 r1 Pak Phli Hospital Nakhonnayok 0010/2557 07/08/2017 06/08/2020 11 10762 4 4 r1 Thanyaburi Hospital Pathum Thani 0011/2557 07/08/2017 06/08/2020 12 10761 4 4 r1 Klong Luang Hospital Pathum Thani 0012/2557 07/08/2017 06/08/2020 13 11141 1 1 P Ban Hong Hospital LamPhun 0014/2557 07/08/2014 06/08/2017 14 11142 1 1 P Li Hospital LamPhun 0015/2557 07/08/2014 06/08/2017 15 11144 1 1 P Pa Sang Hospital LamPhun 0016/2557 07/08/2014 06/08/2017 -

Estimating the Burden of Α-Thalassaemia in Thailand Using A
bioRxiv preprint doi: https://doi.org/10.1101/412718; this version posted September 12, 2018. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY 4.0 International license. 1 Estimating the burden of α-thalassaemia in Thailand using a 2 comprehensive prevalence database for Southeast Asia 3 Carinna Hockham1,2*, Supachai Ekwattanakit3, Samir Bhatt4, Bridget S Penman5, 4 Sunetra Gupta2, Vip Viprakasit3,6 & Frédéric B Piel7 5 6 1The George Institute for Global Health, Sydney, Australia; 2 Evolutionary Ecology of 7 Infectious Disease Group, Department of Zoology, University of Oxford, Oxford, UK, 8 3Thalassaemia Centre, Faculty of Medicine, Siriraj Hospital, Mahidol University, 9 Bangkok, Thailand; 4Department of Infectious Disease Epidemiology, School of Public 10 Health, Imperial College, London, UK; 5Warwick Infectious Disease Epidemiology 11 Research, School of Life Sciences, Warwick University, Coventry, UK; 6Department of 12 Paediatrics, Faculty of Medicine, Siriraj Hospital, Mahidol University, Bangkok, 13 Thailand; 7MRC-PHE Centre for Environment & Health, Department of Epidemiology 14 & Biostatistics, School of Public Health, Imperial College London, London, UK 15 16 *For correspondence: [email protected] 17 18 bioRxiv preprint doi: https://doi.org/10.1101/412718; this version posted September 12, 2018. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY 4.0 International license. 19 Abstract 20 Severe forms of α-thalassaemia, haemoglobin H disease and haemoglobin Bart’s hydrops 21 fetalis, are an important public health concern in Southeast Asia. -

1 2 3 4 5 6 3 H.M. Queen Suthida's Birthday (Holiday - No Classes) 7 8 9 10 11 12 13 7 the Most Holy Trinity 14 15 16 17 18 19 20 8 Classes Begin
ASSUMPTION UNIVERSITY Undergraduate Calendar ACADEMIC YEAR 2020 Semester 1/2020 (June - October 2020) SUN MON TUE WED THU FRI SAT Undergraduate Program 1 2 3 4 5 6 3 H.M. Queen Suthida's Birthday (Holiday - no classes) 7 8 9 10 11 12 13 7 The Most Holy Trinity 14 15 16 17 18 19 20 8 Classes begin JUNE 2020 21 22 23 24 25 26 27 14 The Most Holy Body and Blood of Christ (Corpus Christi) 28 29 30 19 Last day for late registration and adding classes, last day to withdraw TOTAL without record and to have 50% of tuition fees refunded , AVAILABLE 3 4 4 3 3 3 3 Semester 2/2019 Grade Release and The Most Sacred Heart of Jesus CLASSES 23 Retrieve and download registered student namelists via internet for clas attendance checklists SUN MON TUE WED THU FRI SAT 1 2 3 4 5 Asalha Bhucha Day (Holiday - no classes) 5 6 7 8 9 10 11 6 Buddhist Lent Day (Holiday - no classes) 12 13 14 15 16 17 18 7 A Substituted holiday for Asalha Bhucha Day (Holiday - no classes) JULY 2020 JULY 19 20 21 22 23 24 25 8 Submission of Mid-term Examination Papers 26 27 28 29 30 31 28 H.M. King Maha Vajiralongkorn's Birthday (Holiday - no classes) TOTAL 30 Mid-term Examination (till August 07, 2020) AVAILABLE 3 3 2 5 4 4 4 CLASSES SUN MON TUE WED THU FRI SAT 1 8 Classes resume 2 3 4 5 6 7 8 12 H.M. -

Youthquake Evokes the 1932 Revolution and Shakes Thailand's
ISSUE: 2020 No. 127 ISSN 2335-6677 RESEARCHERS AT ISEAS – YUSOF ISHAK INSTITUTE ANALYSE CURRENT EVENTS Singapore | 6 November 2020 Youthquake Evokes the 1932 Revolution and Shakes Thailand’s Establishment Supalak Ganjanakhundee* EXECUTIVE SUMMARY • Grievance and frustration resulting from the government’s authoritarian style, its restrictions on freedom of expression and the dissolution of the Future Forward Party have been accumulating among students and youths in Thailand since the 2014 military coup. • While high school and college students are overwhelmingly represented among participants in the ongoing protests, young people from various other sectors across the country have also joined the demonstrations. • The flash-mob style of demonstration is a venting of anger against the political system, expressed in calls for the resignation of Prime Minister Prayut Chan-ocha, a new Constitution and, more importantly, reform of the Thai monarchy. • The protests are a flashback to the 1932 Revolution, in that they are conveying the message that ordinary people, not the traditional establishment, own the country and have the legitimate right to determine its future course. • In response, the crown and the royalists are using traditional methods of smears and labels to counteract the youths. * Supalak Ganjanakhundee was Visiting Fellow in the Thailand Studies Programme, ISEAS – Yusof Ishak Institute from 1 October 2019 to 30 June 2020. He is the former editor of The Nation (Bangkok). 1 ISSUE: 2020 No. 127 ISSN 2335-6677 INTRODUCTION A number of Thais have gathered annually at Thammasat University’s Tha Phrachan campus and at the 14 October 1973 Memorial site on nearby Ratchadamnoen Avenue to commemorate the student uprising on that date which restored democracy to the country. -

MINISTRY of EDUCATION >>
MINISTRY OF EDUCATION >> √ÕߺŸâÕ”π«¬°“√ΩÉ“¬«‘™“°“√ Deputy Director for Academic and «‘∑¬“≈—¬∫√‘À“√»“ µ√å School of Admininistrative studies ·≈–ª√–°—π§ÿ≥¿“æ°“√»÷°…“ Educational Quality Assurance ºÕ”𫬰“√Ÿâ Director Õ.«’√™—¬ ‡æ™√ ÿ∑∏‘Ï Mr.Weerachai Phetchsuthti º».¥√.ª√“√∂π“ ¬» ÿ¢ Asst.Prof.Dr.Pradtana Yossuck 0 7754 9238 0 5387 3012, 0 5387 3903-6 √ÕߺŸâÕ”π«¬°“√ΩÉ“¬π—°»÷°…“ Deputy Director for Students Affairs Fax 0 5387 3904 ·≈–Õߧå°√ —¡æ—π∏å and Organization Relations www.SAS.mju.ac.th Õ.ª√– “∑æ√ °ÕÕ«¬™—¬Lect.Prasatporn Koauychai ∂“∫—π∫ࡇ擖«‘ “À°‘® Maejo University Institute 0 7754 9238 ¡À“«‘∑¬“≈—¬·¡à‚®â of Business Incubator √ÕߺŸâÕ”π«¬°“√ΩÉ“¬°‘®°“√摇»… Deputy Director for Special Affairs ºŸâÕ”π«¬°“√ ∂“∫—π Director ·≈–°“√»÷°…“ —≠®√ and Assurance Travel Õ.¥√.æ’√°“πµ‘Ï ∫√√‡®‘¥°‘®Lect.Peerakarn Banjerdkit Õ.«’√¿√≥å ‚µ§’√’ Lect.Weeraporn Tokeree 0 5387 3017 Fax 0 5349 8128 0 7754 9238 E-mail : [email protected] ”π—°ß“π∫—≥±‘µ«‘∑¬“≈—¬ Graduate School »π¬Ÿ å«®‘ —¬æ≈—ßß“π Energy Research Center ‡≈¢“πÿ°“√§≥–°√√¡°“√ Secretary ºŸâÕ”π«¬°“√ Director ∫—≥±‘µ»÷°…“ º».¥√.≥—∞«ÿ≤‘ ¥ÿ…Æ’ Asst. Prof.Dr.Natthawud Dussadee º».π√‘π∑√å ∑Õß«‘∑¬“ Asst.Prof.Dr.Narin Thongwittaya 0 5387 5140 Fax 0 5387 5140 0 5387 3553, 0 5387 3608 E-mail : [email protected] 0 5387 3556-8 Fax 0 5349 8133 §≈‘π‘°‡∑§‚π‚≈¬’ Clinic Technology Maejo University E-mail : [email protected] ¡À“«‘∑¬“≈—¬·¡à‚®â ”π—°ø“√å¡¡À“«‘∑¬“≈—¬ Office of University Farm ºÕ”𫬰“√Ÿâ Director ºÕ”𫬰“√Ÿâ Director º».¥√.»‘√‘™—¬ Õÿàπ»√’ àß Asst.Prof.Dr.Sirichai Unsrisong π“¬Õπ—πµå ªîπµ“√—°…å Mr.Anan Pintarak 0 5387 3970-1 Fax 0 5387 3970 0 5387 3072-3 Fax. -
190510 Thailand's Royal Family
Thailand’s royal family Married House of Mahidol Other Chakri House Prince Princess Mahidol Adulyadej Srinagarindra (1892-1929) (1900-1995) Succeeds Princess (Rama VIII) (Rama IX) Queen Galyani King King Sirikit Vadhana Ananda Bhumibol Kitiyakara (1923-2008) Mahidol Adulyadej (b. 1932) (b. 1925) (b. 1927) r. 1935-1946 r. 1946-2016 The princess broke the long-standing tradition of Thai royalty staying out of politics by entering the election. Peter Princess (Rama X) Princess Princess Virayudh Ladd Ubolratana King Maha Sirindhorn Chulabhorn Tishyasarin Jensen Rajakanya Vajiralongkorn (b. 1955) (b. 1957) (b. 1955) (b. 1951) (b. 1951) (b. 1952) m. 1982 m. 1972 div. 1996 div. 1998 First wife CHILDREN Princess Princess Bajrakitiyabha Soamsavali (b. 1978) (b. 1957) CHILDREN CHILDREN m. 1977, div. 1991 Princess Ploypailin Juthavachara Siribhachudhabhorn (b. 1981) (b. 1979) (b. 1982) Second wife Bhumi Vacharaesorn Princess (1983-2004) Sujarinee (b. 1981) Adityadhornkitikhun (b. 1957) (b. 1984) Sirikitiya m. 1994, Chakriwat (b. 1985) div. 1996 (b. 1983) Vatchrawee (b. 1985) Princess Sirivannavari (b. 1987) Third wife Prince Dipangkorn Srirasmi (b. 2005) (b. 1971) m. 2001, div. 2014 Fourth wife Queen Suthida Vajiralongkorn na Ayudhaya • She graduated from Assumption University, a private university in Bangkok, with a bachelor’s degree in communication arts in 2000, according to the Thai Rath newspaper. • She worked as a ight attendant at Thai Airways before joining the protection unit of then-Crown Prince Vajiralongkorn. • Prior to her marriage, she held the rank of general in the Royal Thai Army, having been promoted to the position in December 2016 by King Vajiralongkorn by royal decree. -

Clinical Epidemiology of 7126 Melioidosis Patients in Thailand and the Implications for a National Notifiable Diseases Surveilla
applyparastyle “fig//caption/p[1]” parastyle “FigCapt” View metadata, citation and similar papers at core.ac.uk brought to you by CORE Open Forum Infectious Diseases provided by Apollo MAJOR ARTICLE Clinical Epidemiology of 7126 Melioidosis Patients in Thailand and the Implications for a National Notifiable Diseases Surveillance System Viriya Hantrakun,1, Somkid Kongyu,2 Preeyarach Klaytong,1 Sittikorn Rongsumlee,1 Nicholas P. J. Day,1,3 Sharon J. Peacock,4 Soawapak Hinjoy,2,5 and Direk Limmathurotsakul1,3,6, 1Mahidol-Oxford Tropical Medicine Research Unit (MORU), Faculty of Tropical Medicine, Mahidol University, Bangkok, Thailand, 2 Epidemiology Division, Department of Disease Control, Ministry of Public Health, Nonthaburi, Thailand, 3 Centre for Tropical Medicine and Global Health, Nuffield Department of Clinical Medicine, Old Road Campus, University of Oxford, Oxford, United Kingdom, 4 Department of Medicine, University of Cambridge, Cambridge, United Kingdom, 5 Office of International Cooperation, Department of Disease Control, Ministry of Public Health, Nonthaburi, Thailand, and 6 Department of Tropical Hygiene, Faculty of Tropical Medicine, Mahidol University, Bangkok, Thailand Background. National notifiable diseases surveillance system (NNDSS) data in developing countries are usually incomplete, yet the total number of fatal cases reported is commonly used in national priority-setting. Melioidosis, an infectious disease caused by Burkholderia pseudomallei, is largely underrecognized by policy-makers due to the underreporting of fatal cases via the NNDSS. Methods. Collaborating with the Epidemiology Division (ED), Ministry of Public Health (MoPH), we conducted a retrospec- tive study to determine the incidence and mortality of melioidosis cases already identified by clinical microbiology laboratories nationwide. A case of melioidosis was defined as a patient with any clinical specimen culture positive for B. -

Mortality Risk and Temporal Patterns of Atrial Fibrillation in the Nationwide
medRxiv preprint doi: https://doi.org/10.1101/2021.01.30.21250715; this version posted June 17, 2021. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted medRxiv a license to display the preprint in perpetuity. It is made available under a CC-BY-NC-ND 4.0 International license . Mortality Risk and Temporal Patterns of Atrial Fibrillation in the Nationwide Registry Apiyasawat Short Title: Mortality Risk in Non-paroxysmal AF Sirin Apiyasawat, MD1; Sakaorat Kornbongkotmas, MD2; Ply Chichareon, MD3; Rungroj Krittayaphong, MD4; for the COOL-AF Investigators Links to Affiliations: 1 Faculty of Medicine Ramathibodi Hospital, Mahidol University, Bangkok, Thailand 2 Queen Savang Vadhana Memorial Hospital, Chonburi, Thailand 3 Faculty of Medicine, Prince of Songkla University, Songkla, Thailand 4 Faculty of Medicine, Siriraj Hospital, Mahidol University, Bangkok, Thailand Address for correspondence: Rungroj Krittayaphong, MD Division of Cardiology, Department of Medicine, Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok, Thailand Phone: (66) 2-419-6104; Fax: (66) 2-412-7412, E-mail: [email protected] Word Count: 2299 NOTE: This preprint reports new research that has not been certified by peer review and should not be used to guide clinical practice. medRxiv preprint doi: https://doi.org/10.1101/2021.01.30.21250715; this version posted June 17, 2021. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted medRxiv a license to display the preprint in perpetuity. It is made available under a CC-BY-NC-ND 4.0 International license . -

Sticonpoint June 2019 Edition
June 2019 Issue I am from the Everyone knows me by Maldives. I am International Students my nickname “Willy”. I studying Aeronautics am from Myawaddy, I am a seminarian Kayine, Myanmar. I am in this institution. I staying at Camillian like to swim and dive. 20 years old. I graduated Social Center from Sappawithakhom It’s a passion of mine Prachinburi. Right to explore the School. Before my now, I am studying graduation, I didn’t have underwater beauty. English courses with any idea where to study What I like most about the Business English and what major to take up. One day, Ajarn the college is the hospitality given to me students. I think this Korn Sinchai from STIC Marketing/ by each and everyone on the campus. It institution is good Guidance and Counseling Team introduced is grateful to have a friendly for learning English. St. Theresa International College to grade 12 environment with great personalities. There are many good teachers here and students in our school including myself. Mohamed Shamaail, the environment is a good place to stay. During his presentation, I realized that this is Aeronautics Y1 Nguyen Dinh Minh Quang, what I really want for my tertiary education. Business English Y1 Moreover, the college fees are not expensive My friends call me and I would have the chance to study with Rose. I am 18 years People call me Toby. I real professors. Then, I made the decision to old. I am a Filipino am a German citizen be here at STIC. but was born here in but I grew up in Aung Thu Rain Oo, Aeronautics Y1 Thailand. -

Saturday 5 September 2015
SATURDAY 5 SEPTEMBER 2015 SATURDAY 5 SEPTEMBER Registration Desk 0745-1730 Registration Desk, SECC for Pre-conference Workshop and Course Participants Location: Hall 4, SECC Group Meeting 1000-1700 AMEE Executive Committee Meeting (Closed Meeting) Location: Green Room 10, Back of Hall 4 AMEE-Essential Skills in Medical Education (ESME) Courses Pre-registration is essential and lunch will be provided. 0830-1700 ESME – Essential Skills in Medical Education Location: Argyll I, Crowne Plaza 0845-1630 ESMEA – Essential Skills in Medical Education Assessment Location: Argyll III, Crowne Plaza 0845-1630 RESME – Research Essential Skills in Medical Education Location: Argyll II, Crowne Plaza 0845-1700 ESMESim - Essential Skills in Simulation-based Healthcare Instruction Location: Castle II, Crowne Plaza 0900-1700 ESCEPD – Essential Skills in Continuing Education and Professional Development Location: Castle 1, Crowne Plaza 1000-1330 ESCEL – Essential Skills in Computer-Enhanced Learning Location: Carron 2, SECC Course Pre-registration is essential and lunch will be provided. 0830-1630 ASME-FLAME - Fundamentals of Leadership and Management in Education Location: Castle III, Crowne Plaza Masterclass Pre-registration is essential and lunch will be provided. 0915-1630 MC1 Communication Matters: Demystifying simulation design, debriefing and facilitation practice Kerry Knickle (Standardized Patient Program, Faculty of Medicine, University of Toronto, Canada); Nancy McNaughton, Diana Tabak) University of Toronto, Centre for Research in Education, Standardized -

Age-Related Clinical Outcomes of Patients with Non-Valvular Atrial Fibrillation: Insights from the COOL-AF Registry
Clinical Interventions in Aging Dovepress open access to scientific and medical research Open Access Full Text Article ORIGINAL RESEARCH Age-Related Clinical Outcomes of Patients with Non-Valvular Atrial Fibrillation: Insights from the COOL-AF Registry Rungroj Krittayaphong1 Purpose: We aimed to compare the rate of clinical outcomes among three age groups (<65, Thanita Boonyapiphat2 65–74, and ≥75 years) of adult patients with non-valvular atrial fibrillation (NVAF). Chaiyasith Wongvipaporn3 Patients and Methods: We prospectively enrolled NVAF patients from 27 Thailand Poom Sairat1 medical centers. The following were collected at baseline: demographic data, risk factors, comorbid conditions, laboratory data, and medications. The clinical outcomes were ischemic On behalf of the COOL-AF stroke (IS) or transient ischemic attack (TIA), major bleeding (MB), intracerebral hemor Investigators rhage (ICH), heart failure (HF), and death. All events were adjudicated. Patients were categorized according to age group into three groups; age <65, 65–74, and ≥75 years. 1Division of Cardiology, Department of Results: Among the 3402 patients that were enrolled during 2014–2017, the mean age was 67.4 Medicine, Faculty of Medicine Siriraj ±11.3 years, and 2073 (60.9%) were older. The average follow-up was 25.7±10.6 months. Oral Hospital, Mahidol University, Bangkok, Thailand; 2Division of Cardiology, anticoagulants were given in 75.4% of patients (91.1% of OAC was warfarin). The incidence rate Department of Medicine, Lampang of IS/TIA, MB, ICH, HF, and death was 1.43 (1.17–1.74), 2.11 (1.79–2.48), 0.70 (0.52–0.92), Hospital, Lampang, Thailand; 3Division of Cardiology, Department of Medicine, 3.03 (2.64–3.46), and 3.77 (3.33–4.24) per 100 person-years, respectively. -

Chapter 6 the Expansion New Membership Recruitment Area of Thai Maternal and Child Health Network Under the Royal Patronage 6
Chapter 6 The Expansion New Membership Recruitment Area of Thai Maternal and Child Health Network under the Royal Patronage 6 Thrathip Kolatat, Chantima Charastong At present, Thai Maternal and Child Health Network Board of Committee under the Royal Patronage has established project purposes that meet the principle objective, which is to lower the rate of preterm births. However, the board’s reexamination of the issue reveals the aforementioned strategy can be elevated to be policy-level strategy, the process of which includes setting up the clear strategic targets and public services, as well as considering the differences between service areas. It has also been suggested that personnel in those areas should be the ones coming up with action plans, to successfully reach the ultimate outcome1. Having studied the fundamental patterns of Thai Maternal and Child Health Network’s project management according to national context, the board has established an expansion model, which foresees the project expanding into various other areas, being carried out in a direction towards the expected outcome. Establishing a strategy map in each area begins with strengthening work forces in the provincial level. Since the public health system is directed by Office of Provincial Chief Medical Officer, which provides management and supports to community and district health promotion hospitals, not the general and regional hospitals. Thus, if there is to be an integration of both health promotion and treatment, an operational conference is essential. The conference would allow idea sharing and discussion between the multidisciplinary involved, namely obstetricians, pediatricians, general practitioners, registered nurses (from prenatal clinics, delivery rooms, emergency rooms, neonatal intensive care unit, neonatal wards and follow-up clinics, etc.), as well as public health technical officers, social medicine officers from community hospitals, general hospitals and regional hospitals.