Mortality Risk and Temporal Patterns of Atrial Fibrillation in the Nationwide
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
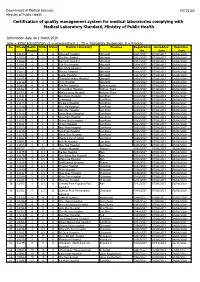
Certification of Quality Management System for Medical Laboratories Complying with Medical Laboratory Standard, Ministry of Public Health
Department of Medical Sciences F0715102 Ministry of Public Health Certification of quality management system for medical laboratories complying with Medical Laboratory Standard, Ministry of Public Health Information date on 1 March 2019 new = initial accreditation, r1 = reassessment 1 , TS = Temporary Suspension, P = Process No. HCode Health RMSc Status Medical Laboratory Province Registration Accredited Expiration Region No. Date Date 1 10673 2 2 r1 Uttaradit Hospital Uttaradit 0001/2557 07/08/2017 06/08/2020 2 11159 2 2 r1 Tha Pla Hospital Uttaradit 0002/2557 07/08/2017 06/08/2020 3 11160 2 2 r1 Nam Pat Hospital Uttaradit 0003/2557 07/08/2017 06/08/2020 4 11161 2 2 r1 Fak Tha Hospital Uttaradit 0004/2557 07/08/2017 06/08/2020 5 11162 2 2 r1 Ban Khok Hospital Uttaradit 0005/2557 07/08/2017 06/08/2020 6 11163 2 2 r1 Phichai Hospital Uttaradit 0006/2557 07/08/2017 06/08/2020 7 11164 2 2 r1 Laplae Hospital Uttaradit 0007/2557 07/08/2017 06/08/2020 8 11165 2 2 r1 ThongSaenKhan Hospital Uttaradit 0008/2557 07/08/2017 06/08/2020 9 11158 2 2 r1 Tron Hospital Uttaradit 0009/2557 07/08/2017 06/08/2020 10 10863 4 4 r1 Pak Phli Hospital Nakhonnayok 0010/2557 07/08/2017 06/08/2020 11 10762 4 4 r1 Thanyaburi Hospital Pathum Thani 0011/2557 07/08/2017 06/08/2020 12 10761 4 4 r1 Klong Luang Hospital Pathum Thani 0012/2557 07/08/2017 06/08/2020 13 11141 1 1 P Ban Hong Hospital LamPhun 0014/2557 07/08/2014 06/08/2017 14 11142 1 1 P Li Hospital LamPhun 0015/2557 07/08/2014 06/08/2017 15 11144 1 1 P Pa Sang Hospital LamPhun 0016/2557 07/08/2014 06/08/2017 -

Bangkok Anesthesia Regional Training Center
RoleRole ofof BARTCBARTC (Bangkok(Bangkok AnesthesiaAnesthesia RegionalRegional TrainingTraining Center)Center) IInn cooperationcooperation inin educationeducation andand trainingtraining inin developingdeveloping countriescountries ProfProf TharaThara TritrakarnTritrakarn DirectorDirector ofof BARTCBARTC 14th WCA, Cape Town, South Africa, 3/1/2008 Oslo Center, Norway, 12/1/2008 ShortageShortage ofof anesthesiologistsanesthesiologists AA worldwideworldwide problemsproblems MoreMore seriousserious inin developingdeveloping poorpoor countriescountries MarkedMarked variationvariation amongamong countriescountries EconomyEconomy - Most important determining factors - Three levels of wealth & health - Rich countries (per capita GNP > $ 10,000) - Medium to low (GNP $ 1,000-10,000) - Poor countries (GNP < $ 1,000) RichRich && MediumMedium countriescountries GNPGNP PeoplePeople NumberNumber PeoplePeople perper capitacapita perper ofof perper (US(US $)$) doctordoctor anesthetistsanesthetists anesthetistanesthetist USA 33,799 387 23,300 11,500 Japan 34,715 522 4,229 20,000 Singapore 22,710 667 150 26,600 Hong Kong 23,597 772 150 40,000 Australia 19,313 2170 10,000 Malaysia 3,248 1,477 250 88,000 Thailand 1,949 2,461 500 124,000 Philippines 1,048 1,016 1176 64,600 MediumMedium && PoorPoor CountriesCountries GNPGNP PeoplePeople NumberNumber PeoplePeople perper capitacapita perper ofof perper (US(US $)$) doctordoctor anesthetistsanesthetists anesthetistanesthetist Indonesia 617 6,7866,786 350 591,000591,000 Pakistan 492 2,0002,000 400 340,000340,000 -

January 08-11 Pp01
ANDAMAN Edition Volume 18 Issue 2 January 8 - 14, 2011 Daily news at www.phuketgazette.net 25 Baht Booze ban hits park tourists Teen stabbing sparks alcohol ban in national parks By Atchaa Khamlo derstand and have given us very Standard procedure is for staff good co-operation. The situation to ask people trying to bring alco- THE directors of several national is under control,” he said. hol into the park to leave it with parks in the Andaman region say Most of those warned about officers during their visit, he said. they have received good compli- drinking in the park were Thais, “We prefer to ask people for ance with the ban on alcohol in- but a few were foreigners. their co-operation rather than side parks that came into effect “As the park is quite expansive, threaten them with punishment. It on December 27. sometimes people might be drink- seems our public relations cam- Natural Resources and Envi- ing inside without our being aware paign is going well, as most people ronment Minister Suwit Khunkitti of it,” he added. just drink Coke or water,” he said. issued the ban immediately follow- Two signs declaring the park “Most foreigners understand ing the December 26 stabbing an alcohol prohibition zone are quite well. Not many of them drink murder of a student by a group of now being constructed and should whiskey, but some like to drink drunken schoolmates camping at go up at both entrances very soon, beer. But they don’t seem to have Khao Yai National Park in Surat along with a third sign to go up in any problem with alcohol being Thani. -

Estimating the Burden of Α-Thalassaemia in Thailand Using A
bioRxiv preprint doi: https://doi.org/10.1101/412718; this version posted September 12, 2018. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY 4.0 International license. 1 Estimating the burden of α-thalassaemia in Thailand using a 2 comprehensive prevalence database for Southeast Asia 3 Carinna Hockham1,2*, Supachai Ekwattanakit3, Samir Bhatt4, Bridget S Penman5, 4 Sunetra Gupta2, Vip Viprakasit3,6 & Frédéric B Piel7 5 6 1The George Institute for Global Health, Sydney, Australia; 2 Evolutionary Ecology of 7 Infectious Disease Group, Department of Zoology, University of Oxford, Oxford, UK, 8 3Thalassaemia Centre, Faculty of Medicine, Siriraj Hospital, Mahidol University, 9 Bangkok, Thailand; 4Department of Infectious Disease Epidemiology, School of Public 10 Health, Imperial College, London, UK; 5Warwick Infectious Disease Epidemiology 11 Research, School of Life Sciences, Warwick University, Coventry, UK; 6Department of 12 Paediatrics, Faculty of Medicine, Siriraj Hospital, Mahidol University, Bangkok, 13 Thailand; 7MRC-PHE Centre for Environment & Health, Department of Epidemiology 14 & Biostatistics, School of Public Health, Imperial College London, London, UK 15 16 *For correspondence: [email protected] 17 18 bioRxiv preprint doi: https://doi.org/10.1101/412718; this version posted September 12, 2018. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY 4.0 International license. 19 Abstract 20 Severe forms of α-thalassaemia, haemoglobin H disease and haemoglobin Bart’s hydrops 21 fetalis, are an important public health concern in Southeast Asia. -

Resistance Patterns Selected by Nevirapine Vs
Resistance Patterns Selected by Nevirapine vs. Efavirenz in HIV-Infected Patients Failing First-Line Antiretroviral Treatment: A Bayesian Analysis Nicole Ngo-Giang-Huong1,2,3*, Gonzague Jourdain1,2,3, Billy Amzal1,2, Pensiriwan Sang-a-gad4, Rittha Lertkoonalak5, Naree Eiamsirikit6, Somboon Tansuphasawasdikul7, Yuwadee Buranawanitchakorn8, Naruepon Yutthakasemsunt9, Sripetcharat Mekviwattanawong10, Kenneth McIntosh3,11, Marc Lallemant1,2,3, for the Program for HIV Prevention and Treatment (PHPT) study group" 1 Institut de Recherche pour le De´veloppement (IRD) UMI 174 - PHPT, Marseilles, France, 2 Department of Medical Technology, Faculty of Associated Medical Sciences, Chiang Mai University, Chiang Mai, Thailand, 3 Department of Immunology and Infectious Diseases, Harvard School of Public Health, Boston, Massachusetts, United States of America, 4 Ratchaburi Hospital, Ratchaburi, Thailand, 5 Maharat Nakonratchasima Hospital, Nakonratchasima, Thailand, 6 Samutprakarn Hospital, Samutprakarn, Thailand, 7 Buddhachinaraj Hospital, Pitsanuloke, Thailand, 8 Chiang Kham Hospital, Chiang Kham, Thailand, 9 Nong Khai Hospital, Nong Khai, Thailand, 10 Pranangklao Hospital, Bangkok, Thailand, 11 Division of Infectious Diseases, Children’s Hospital, Boston, Massachusetts, United States of America Abstract Background: WHO recommends starting therapy with a non-nucleoside reverse transcriptase inhibitor (NNRTI) and two nucleoside reverse transcriptase inhibitors (NRTIs), i.e. nevirapine or efavirenz, with lamivudine or emtricitabine, plus zidovudine or -

Clinical Epidemiology of 7126 Melioidosis Patients in Thailand and the Implications for a National Notifiable Diseases Surveilla
applyparastyle “fig//caption/p[1]” parastyle “FigCapt” View metadata, citation and similar papers at core.ac.uk brought to you by CORE Open Forum Infectious Diseases provided by Apollo MAJOR ARTICLE Clinical Epidemiology of 7126 Melioidosis Patients in Thailand and the Implications for a National Notifiable Diseases Surveillance System Viriya Hantrakun,1, Somkid Kongyu,2 Preeyarach Klaytong,1 Sittikorn Rongsumlee,1 Nicholas P. J. Day,1,3 Sharon J. Peacock,4 Soawapak Hinjoy,2,5 and Direk Limmathurotsakul1,3,6, 1Mahidol-Oxford Tropical Medicine Research Unit (MORU), Faculty of Tropical Medicine, Mahidol University, Bangkok, Thailand, 2 Epidemiology Division, Department of Disease Control, Ministry of Public Health, Nonthaburi, Thailand, 3 Centre for Tropical Medicine and Global Health, Nuffield Department of Clinical Medicine, Old Road Campus, University of Oxford, Oxford, United Kingdom, 4 Department of Medicine, University of Cambridge, Cambridge, United Kingdom, 5 Office of International Cooperation, Department of Disease Control, Ministry of Public Health, Nonthaburi, Thailand, and 6 Department of Tropical Hygiene, Faculty of Tropical Medicine, Mahidol University, Bangkok, Thailand Background. National notifiable diseases surveillance system (NNDSS) data in developing countries are usually incomplete, yet the total number of fatal cases reported is commonly used in national priority-setting. Melioidosis, an infectious disease caused by Burkholderia pseudomallei, is largely underrecognized by policy-makers due to the underreporting of fatal cases via the NNDSS. Methods. Collaborating with the Epidemiology Division (ED), Ministry of Public Health (MoPH), we conducted a retrospec- tive study to determine the incidence and mortality of melioidosis cases already identified by clinical microbiology laboratories nationwide. A case of melioidosis was defined as a patient with any clinical specimen culture positive for B. -

Saturday 5 September 2015
SATURDAY 5 SEPTEMBER 2015 SATURDAY 5 SEPTEMBER Registration Desk 0745-1730 Registration Desk, SECC for Pre-conference Workshop and Course Participants Location: Hall 4, SECC Group Meeting 1000-1700 AMEE Executive Committee Meeting (Closed Meeting) Location: Green Room 10, Back of Hall 4 AMEE-Essential Skills in Medical Education (ESME) Courses Pre-registration is essential and lunch will be provided. 0830-1700 ESME – Essential Skills in Medical Education Location: Argyll I, Crowne Plaza 0845-1630 ESMEA – Essential Skills in Medical Education Assessment Location: Argyll III, Crowne Plaza 0845-1630 RESME – Research Essential Skills in Medical Education Location: Argyll II, Crowne Plaza 0845-1700 ESMESim - Essential Skills in Simulation-based Healthcare Instruction Location: Castle II, Crowne Plaza 0900-1700 ESCEPD – Essential Skills in Continuing Education and Professional Development Location: Castle 1, Crowne Plaza 1000-1330 ESCEL – Essential Skills in Computer-Enhanced Learning Location: Carron 2, SECC Course Pre-registration is essential and lunch will be provided. 0830-1630 ASME-FLAME - Fundamentals of Leadership and Management in Education Location: Castle III, Crowne Plaza Masterclass Pre-registration is essential and lunch will be provided. 0915-1630 MC1 Communication Matters: Demystifying simulation design, debriefing and facilitation practice Kerry Knickle (Standardized Patient Program, Faculty of Medicine, University of Toronto, Canada); Nancy McNaughton, Diana Tabak) University of Toronto, Centre for Research in Education, Standardized -

Switching HIV Treatment in Adults Based on CD4 Count Versus Viral Load Monitoring: a Randomized, Non- Inferiority Trial in Thailand
Switching HIV Treatment in Adults Based on CD4 Count Versus Viral Load Monitoring: A Randomized, Non- Inferiority Trial in Thailand Gonzague Jourdain1,2,3, Sophie Le Cœur1,2,3,4, Nicole Ngo-Giang-Huong1,2,3, Patrinee Traisathit5, Tim R. Cressey1,2,3, Federica Fregonese1, Baptiste Leurent1, Intira J. Collins1,3, Malee Techapornroong6, Sukit Banchongkit7, Sudanee Buranabanjasatean8, Guttiga Halue9, Ampaipith Nilmanat10, Nuananong Luekamlung11, Virat Klinbuayaem12, Apichat Chutanunta13, Pacharee Kantipong14, Chureeratana Bowonwatanuwong15, Rittha Lertkoonalak16, Prattana Leenasirimakul17, Somboon Tansuphasawasdikul18, Pensiriwan Sang-a-gad19, Panita Pathipvanich20, Srisuda Thongbuaban21, Pakorn Wittayapraparat22, Naree Eiamsirikit23, Yuwadee Buranawanitchakorn24, Naruepon Yutthakasemsunt25, Narong Winiyakul26, Luc Decker1,2, Sylvaine Barbier1, Suporn Koetsawang27, Wasna Sirirungsi2, Kenneth McIntosh3,28, Sombat Thanprasertsuk29, Marc Lallemant1,2,3*, PHPT-3 study team 1 Unite´ Mixte Internationale 174, Institut de Recherche pour le De´veloppement (IRD)-Programs for HIV Prevention and Treatment (PHPT), Chiang Mai, Thailand, 2 Department of Medical Technology, Faculty of Associated Medical Sciences, Chiang Mai University, Chiang Mai, Thailand, 3 Department of Immunology and Infectious Diseases, Harvard School of Public Health, Boston, Massachusetts, United States of America, 4 Unite´ Mixte de Recherche 196, Centre Franc¸ais de la Population et du De´veloppement, (INED-IRD-Paris V University), Paris, France, 5 Department of Statistics, Faculty -

Age-Related Clinical Outcomes of Patients with Non-Valvular Atrial Fibrillation: Insights from the COOL-AF Registry
Clinical Interventions in Aging Dovepress open access to scientific and medical research Open Access Full Text Article ORIGINAL RESEARCH Age-Related Clinical Outcomes of Patients with Non-Valvular Atrial Fibrillation: Insights from the COOL-AF Registry Rungroj Krittayaphong1 Purpose: We aimed to compare the rate of clinical outcomes among three age groups (<65, Thanita Boonyapiphat2 65–74, and ≥75 years) of adult patients with non-valvular atrial fibrillation (NVAF). Chaiyasith Wongvipaporn3 Patients and Methods: We prospectively enrolled NVAF patients from 27 Thailand Poom Sairat1 medical centers. The following were collected at baseline: demographic data, risk factors, comorbid conditions, laboratory data, and medications. The clinical outcomes were ischemic On behalf of the COOL-AF stroke (IS) or transient ischemic attack (TIA), major bleeding (MB), intracerebral hemor Investigators rhage (ICH), heart failure (HF), and death. All events were adjudicated. Patients were categorized according to age group into three groups; age <65, 65–74, and ≥75 years. 1Division of Cardiology, Department of Results: Among the 3402 patients that were enrolled during 2014–2017, the mean age was 67.4 Medicine, Faculty of Medicine Siriraj ±11.3 years, and 2073 (60.9%) were older. The average follow-up was 25.7±10.6 months. Oral Hospital, Mahidol University, Bangkok, Thailand; 2Division of Cardiology, anticoagulants were given in 75.4% of patients (91.1% of OAC was warfarin). The incidence rate Department of Medicine, Lampang of IS/TIA, MB, ICH, HF, and death was 1.43 (1.17–1.74), 2.11 (1.79–2.48), 0.70 (0.52–0.92), Hospital, Lampang, Thailand; 3Division of Cardiology, Department of Medicine, 3.03 (2.64–3.46), and 3.77 (3.33–4.24) per 100 person-years, respectively. -

Chapter 6 the Expansion New Membership Recruitment Area of Thai Maternal and Child Health Network Under the Royal Patronage 6
Chapter 6 The Expansion New Membership Recruitment Area of Thai Maternal and Child Health Network under the Royal Patronage 6 Thrathip Kolatat, Chantima Charastong At present, Thai Maternal and Child Health Network Board of Committee under the Royal Patronage has established project purposes that meet the principle objective, which is to lower the rate of preterm births. However, the board’s reexamination of the issue reveals the aforementioned strategy can be elevated to be policy-level strategy, the process of which includes setting up the clear strategic targets and public services, as well as considering the differences between service areas. It has also been suggested that personnel in those areas should be the ones coming up with action plans, to successfully reach the ultimate outcome1. Having studied the fundamental patterns of Thai Maternal and Child Health Network’s project management according to national context, the board has established an expansion model, which foresees the project expanding into various other areas, being carried out in a direction towards the expected outcome. Establishing a strategy map in each area begins with strengthening work forces in the provincial level. Since the public health system is directed by Office of Provincial Chief Medical Officer, which provides management and supports to community and district health promotion hospitals, not the general and regional hospitals. Thus, if there is to be an integration of both health promotion and treatment, an operational conference is essential. The conference would allow idea sharing and discussion between the multidisciplinary involved, namely obstetricians, pediatricians, general practitioners, registered nurses (from prenatal clinics, delivery rooms, emergency rooms, neonatal intensive care unit, neonatal wards and follow-up clinics, etc.), as well as public health technical officers, social medicine officers from community hospitals, general hospitals and regional hospitals. -

Outcome Disparities by Insurance Plan and Educational Attainment In
medRxiv preprint doi: https://doi.org/10.1101/2021.04.25.21256068; this version posted April 28, 2021. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted medRxiv a license to display the preprint in perpetuity. It is made available under a CC-BY-NC-ND 4.0 International license . Outcome Disparities by Insurance Plan and Educational Attainment in Patients with Atrial Fibrillation Apiyasawat Short Title: Education Disparities and AF Outcomes Sirin Apiyasawat, MD1; Tomon Thongsri, MD2, Kulyot Jongpiputvanich, MD3, Rungroj Krittayaphong, MD4; for the COOL-AF Investigators 1 Faculty of Medicine, Ramathibodi Hospital, Mahidol University, Bangkok, Thailand 2 Buddhachinaraj Hospital, Phitsanulok, Thailand 3 Faculty of Medicine, Vajira Hospital, Navamindradhiraj University, Bangkok, Thailand 4 Faculty of Medicine, Siriraj Hospital, Mahidol University, Bangkok, Thailand Address for correspondence: Rungroj Krittayaphong, MD Division of Cardiology, Department of Medicine, Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok, Thailand Phone: (66) 2-419-6104; Fax: (66) 2-412-7412, E-mail: [email protected] Word Counts: 2990 Keywords: atrial fibrillation, health insurance, education, mortality, registry Apiyasawat,NOTE: This preprint et al. reports new research that has not been certified1 by peer review Educationand should not Disparities be used to guide and clinical AF Outcomes practice. medRxiv preprint doi: https://doi.org/10.1101/2021.04.25.21256068; this version posted April 28, 2021. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted medRxiv a license to display the preprint in perpetuity. -

Geographic Dynamics of Viral Encephalitis in Thailand Timothy Jensen Henrich Yale University
Yale University EliScholar – A Digital Platform for Scholarly Publishing at Yale Yale Medicine Thesis Digital Library School of Medicine 2004 Geographic dynamics of viral encephalitis in Thailand Timothy Jensen Henrich Yale University Follow this and additional works at: http://elischolar.library.yale.edu/ymtdl Recommended Citation Henrich, Timothy Jensen, "Geographic dynamics of viral encephalitis in Thailand" (2004). Yale Medicine Thesis Digital Library. 2707. http://elischolar.library.yale.edu/ymtdl/2707 This Open Access Thesis is brought to you for free and open access by the School of Medicine at EliScholar – A Digital Platform for Scholarly Publishing at Yale. It has been accepted for inclusion in Yale Medicine Thesis Digital Library by an authorized administrator of EliScholar – A Digital Platform for Scholarly Publishing at Yale. For more information, please contact [email protected]. GEOGRAPHIC DYNAMICS OF VIRAL : ElfCEfiHAUTIS IN THAILAND : Timothy Jensen Hen rich Yale University YALE UNIVERSITY CUSHING/WHITNEY MEDICAL LIBRARY Permission to photocopy or microfilm processing of this thesis for the purpose of individual scholarly consultation or reference is hereby granted by the author. This permission is not to be interpreted as affecting publication of this work or otherwise placing it in the public domain, and the author reserves all rights of ownership guaranteed under common law protection of unpublished manuscripts. Signature of Author Date Digitized by the Internet Archive in 2017 with funding from The National Endowment